29.
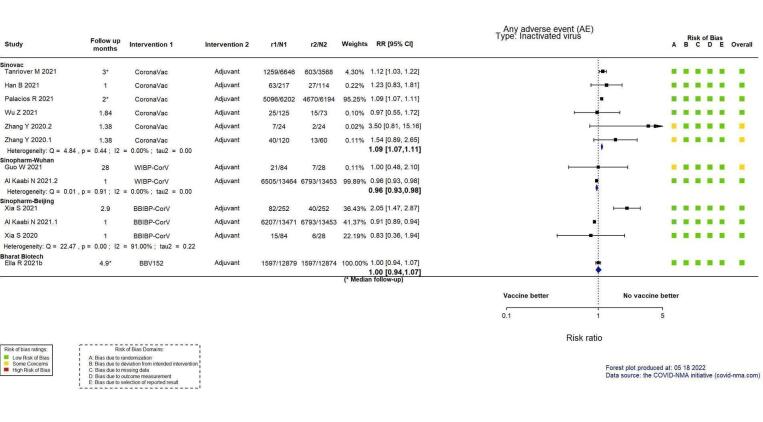
Analysis 3.1.7: inactivated virus vaccine. Outcome: any adverse event (AE).
Han B 2021 and Xia 2021 included only participants 3 to 17 years of age (Han 2021; Xia 2021). Wu Z 2021 included only participants 60 years of age and older (Wu 2021a).
Wu Z 2021 reports data for phase 1 and 2 (Wu 2021a), Al Kaabi 2021.1 and Al Kaabi N 2021.2 refer to two different comparisons from the same report (Al Kaabi 2021). Zhang 2020.1 and Zhang 2020.2 refers to two different comparisons from the same report (Zhang 2021).