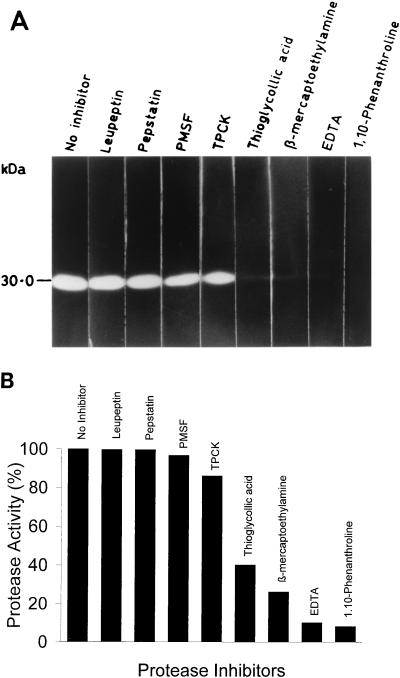
FIG. 4.
Effect of protease inhibitors on protease activity. (A) Immunoprecipitated PVHSP28 from an in vitro translation reaction was incubated with protease inhibitors for 30 min at 37°C and then electrophoresed on a gelatin gel. Individual lanes of the gel were kept with the indicated inhibitors in Tris-Cl buffer, pH 7.4. Gels were developed as described in Materials and Methods. (B) Hydrolysis of succinylated casein by PVHSP28 was studied in the presence of inhibitors. Each assay was carried out in triplicate, and the results shown are the mean percent inhibition, taking 0% inhibition as that for the control without any inhibitor. The concentrations of protease inhibitors were as follows: 1,10-phenanthroline, 1 mM; EDTA, 1 mM; β-mercaptoethylamine, 1 mM; thioglycolic acid, 1 mM; TPCK, 0.1 μg/μl; PMSF, 1 mM; pepstatin, 10 μM; and leupeptin, 10 μM.