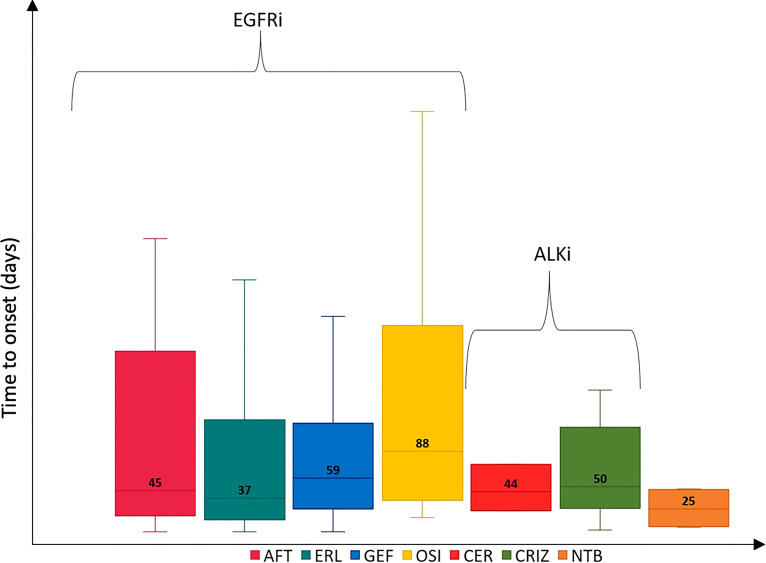
Figure 2.
Time to onset of Sicilian ADRs related to TKIs approved for NSCLC. ADR, adverse drug reaction; AFT, afatinib; ALEC, alectinib; ALKi, anaplastic lymphoma kinase inhibitors; BRG, brigatinib; CER, ceritinib; CRIZ, crizotinib; EGFRi, epidermal growth factor receptor inhibitors; ERL, erlotinib; GEF, gefitinib; LORL, lorlatinib; NSCLC, non-small cell lung cancer; NTB, nintedanib; OSI, osimertinib; TKIs, tyrosine kinase inhibitors.