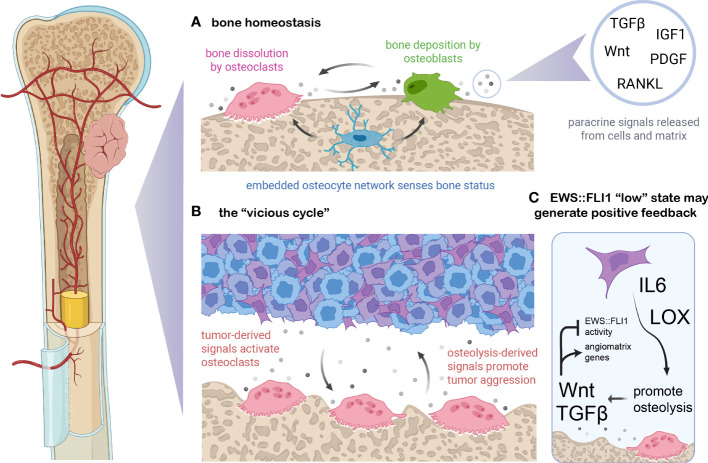
Figure 3.
EWS::FLI1 activity is regulated by signals in the osteolytic bone tumor microenvironment. (A) Bone is constantly remodeled and homeostasis is maintained by osteoclasts (which resorb bone through protease and proton secretion), osteoblasts (which deposit mineralized, collagenous matrix), and osteoblast-derived osteocytes which form a network within bone and modulate osteoclast and osteoblast activity (110). Soluble signals exist either embedded in the bone matrix (and released upon dissolution) or generated via paracrine secretion from bone cells. These signals couple remodeling to deposition and shifts in these signals can either balance bone remodeling, favor deposition during periods of bone growth, or favor osteolysis during regression or calcium deficiency. (B) Ewing sarcoma cells can secrete numerous signals which dysregulate this balance, including osteoblast-activating factors such as RANKL, IL6, TNF-alpha, and LOX (109, 111–113). In turn, the dissolution of bone matrix releases embedded growth factors such Wnts, TGF-beta, PDGFs, FGFs, and IGF1 which can promote tumor growth and/or metastasis (111). (C) EWS::FLI1 “low” cells may generate a positive feedback loop with the osteolytic “vicious cycle”. Recent findings suggest that bone-derived signals such as Wnt and TGF-beta partially antagonize EWS::FLI1 activity, promoting an EWS::FLI1 “low” transcriptional state that upregulates mesenchymal-identify genes including pro-metastatic ECM molecules and angiogenesis-inducing genes (23). EWS::FLI1 antagonism also induces expression of LOX and IL-6, known promoters of osteolysis (17, 35, 114). Figure created with BioRender.com.