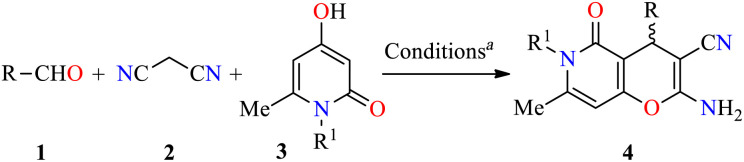
Three-component synthesis and anticancer evaluation of pyrano[3,2-c]pyridones 4.
| ||||
|---|---|---|---|---|
| Compound | R | R1 | Yield 4 (%) | IC50b (μM) |
| HeLa | ||||
| 4a | 3-Br-4-NMe2C6H3 | Me | 83 | 0.33 ± 0.06 |
| 4b | 3-Br-4,5-(MeO)2C6H2 | Me | 87 | 0.58 ± 0.14 |
| 4c | 3-Br-4-EtO-5-MeOC6H2 | Me | 88 | 1.08 ± 0.8 |
| 4d | 3-Br-4-OH-5-MeOC6H2 | Me | 75 | 2.67 ± 1.1 |
| 4e | 3-Br-4-AcO-5-MeOC6H2 | Me | 83 | 3.50 ± 1.3 |
| 4f | 3-Br-4-F-C6H3 | Me | 84 | 6.33 ± 1.1 |
| 4g | 3-BrC6H4 | Me | 97 | 6.50 ± 1.3 |
| 4h | 3,4-(Cl)2C6H3 | Me | 98 | 18.3 ± 2.9 |
| 4i | 3,4,5-(MeO)3C6H2 | Me | 97 | 43.3 ± 5.1 |
| 4j | 4-iPrC6H4 | Me | 81 | >100 |
| 4k | 3-NO2C6H4 | Me | 97 | 35.0 ± 21.8 |
| 4l | 3,4,5-(MeO)3C6H2 | 3,4-Dimethoxyphenethyl | 80 | 22.7 ± 6.4 |
Reaction conditions: aldehyde 1 (0.8 mmol), malononitrile 2 (0.8 mmol), 4-hydroxy-1,6-dimethylpyridin-2(1H)-one 3 (0.8 mmol), triethylamine (45 mol%), EtOH (3 mL), reflux, 50 min.
Compound concentration required to reduce HeLa cell viability by 50% after 48 h treatment relative to 100% DMSO control as assessed with the MTT assay. Data shown are average ± SD of three independent experiments.
