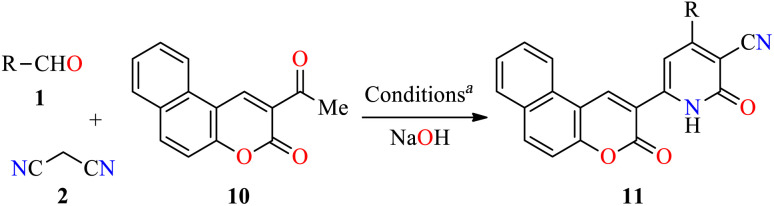
Four-component synthesis and anticancer evaluation of chromene-based 2(1H)-pyridones 11.
| ||||
|---|---|---|---|---|
| Compound | R | Yield 11 (%) | IC50c (μM) | |
| HePG2 | MCF-7 | |||
| 11a | C6H5 | 87 | 77.6 | 78.3 |
| 11b | 4-OHC6H4 | 85 | 58.1 | 59.9 |
| 11c | 4-MeOC6H4 | 79 | 53.6 | 56.3 |
| 11d | 4-Me2NC6H4 | 71 | — | — |
| 5-Fluorouracilb | — | — | 9.30 | 13.1 |
Reaction conditions: aldehyde 1 (1 mmol), malononitrile 2 (1.5 mmol), 2-acetyl-3H-benzo[f]chromen-3-one 10 (1 mmol), NaOH (1.5 mmol), 75 °C, 45 min.
Standard drug for the study.
Compound concentration required to reduce HePG2 and MCF-7 cells viability by 50% after 48 h treatment relative to 100% DMSO control as assessed with the MTT assay.
