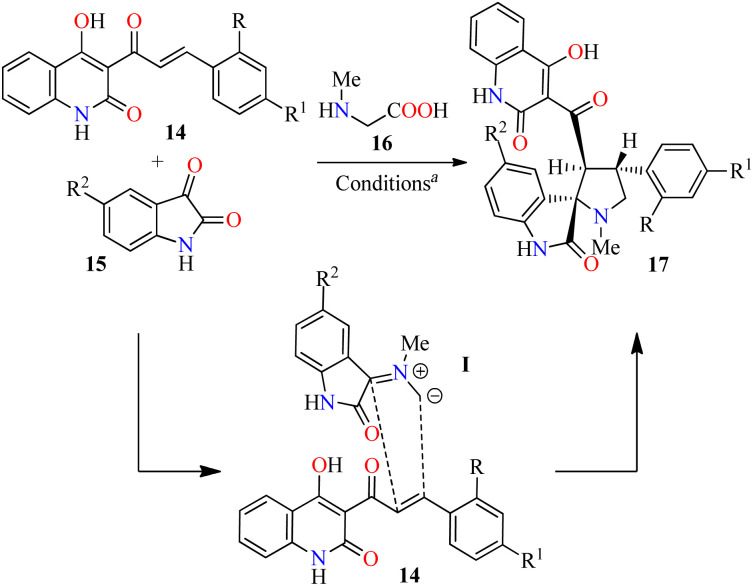
Three-component synthesis and anticancer evaluation of spiropyrrolidine derivatives 17.
| ||||||
|---|---|---|---|---|---|---|
| Compound | R | R1 | R2 | Yield 17 (%) | IC50c (μM) | |
| HCT-116 | HeLa | |||||
| 17a | H | H | H | 92 | 83.2 ± 0.42 | 15.1 ± 0.18 |
| 17b | Cl | H | H | 88 | — | 10.7 ± 0.15 |
| 17c | H | Me | H | 62 | 9.6 ± 0.14 | 28.4 ± 0.30 |
| 17d | H | H | NO2 | 54 | 9.3 ± 0.12 | 16.7 ± 0.14 |
| 17e | H | Me | NO2 | 46 | 10.9 ± 0.18 | 25.5 ± 0.28 |
| 17f | H | Cl | H | 87 | 12.0 ± 0.20 | 34.6 ± 0.17 |
| 17g | H | Br | H | 82 | — | — |
| Doxorubicinb | — | — | — | — | 2.4 ± 0.10 | 1.8 ± 0.05 |
Reaction conditions: 4-hydroxyquinoline 14 (1.5 mmol), isatine 15 (1.5 mmol), sarcosine 16 (1.5 mmol), MeOH/H2O (2 : 1, v/v), reflux, 2.5–3.0 h.
Standard drug for the study.
Compound concentration required to reduce HCT-116 and HeLa cells viability by 50% after 48 h treatment relative to 100% DMSO control as assessed with the MTT assay. Data shown are average ± SD of two independent experiments.
