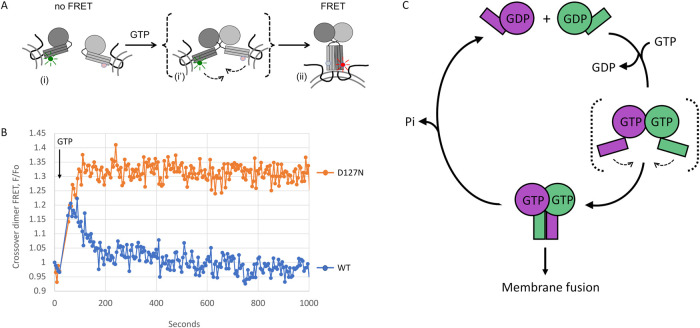
FIGURE 4:
D127N DATL is blocked in dimer disassembly and model. (A) Schematic of FRET assay for dimerization and crossover by full-length membrane-anchored DATL. GTP-induced G domain dimerization and concomitant conformational changes bring donor dye- and acceptor dye-labeled 3HBs of opposing DATLs (i) into close parallel alignment to induce FRET (ii). A transient intermediate (i’) dimerized only through the G domain is shown in brackets. (B) D127N DATL is defective in dimer disassembly. Change in FRET acceptor fluorescence was measured by first establishing a baseline followed by 5 µM GTP addition at the timepoint indicated. FRET donor- and acceptor-labeled proteins were incorporated into vesicles at a 1:1000 protein/lipid ratio. Assays used a 1:2 donor/acceptor ratio at 2 µM final protein concentration. Data shown are the average of two technical replicate traces. (C) Model. GTP binding by ATL in opposing membranes induces trans G domain dimerization and a crossover conformational change that drives membrane fusion. GTP hydrolysis, induced only after stable dimerization has occurred, triggers subsequent dimer disassembly to enable reuse of ATL subunits for multiple rounds of fusion catalysis. A transient intermediate dimerized only through the G domain, which is speculated to initiate membrane tethering, is shown in brackets.