Figure 1.
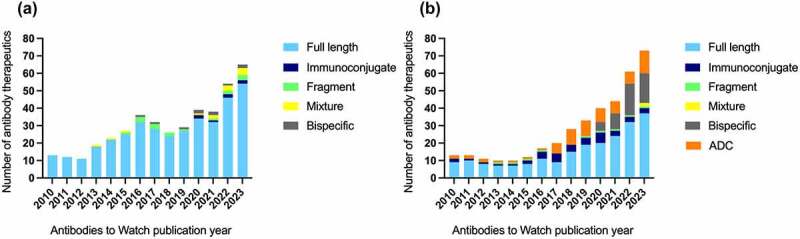
Format and number of antibody therapeutics in late-stage clinical studies from 2010–2023*. (a) Format and number of antibodies in late-stage clinical studies for diseases other than cancer (i.e., all non-cancer indications). Antibodies that entered late-stage studies for COVID-19 during 2020–2022 were excluded to enable accurate comparisons to data from previous years. (b) Format and number of antibodies in late-stage clinical studies for cancer. As defined here, ADCs are antibody-derived proteins conjugated to a small molecule drug through a linker, while immunoconjugates are antibody-derived proteins fused or conjugated to any other biologically relevant modality, e.g., protein, radioisotope. Data were derived from the ‘Antibodies to Watch’ articles;1–13 data for each article are collected at the end of the previous year. *Data for 2023 is as of October 1, 2022. Abbreviations: ADC, antibody–drug conjugate.
