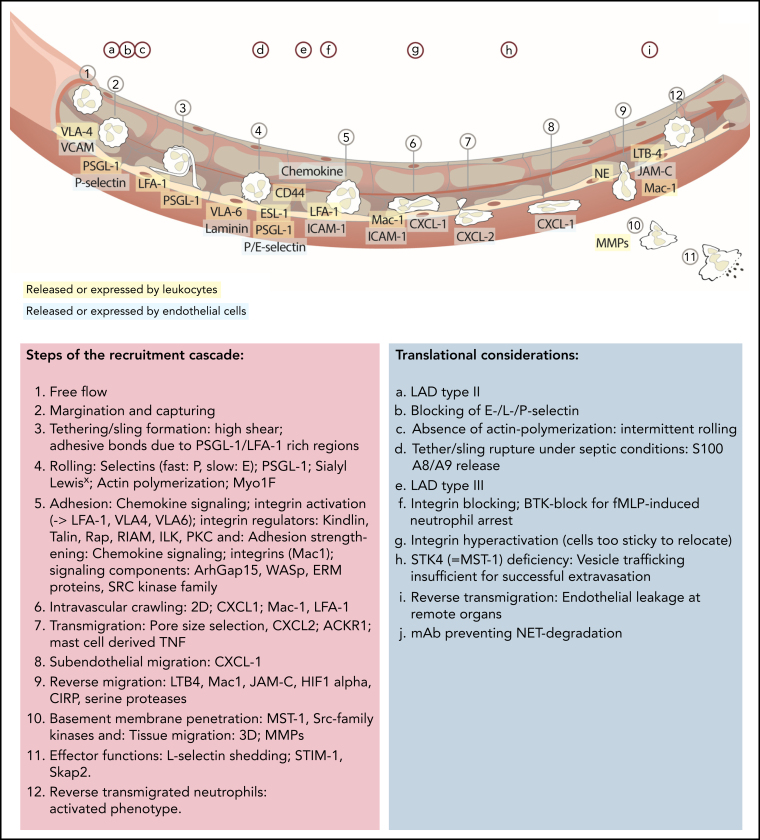
Figure 1.
Updated leukocyte recruitment cascade and translational implications. Free-flowing neutrophils are captured by transient PSGL-1–selectin and VLA-4–VCAM/VLA-6–laminin interactions. Rolling (fast: P-selectin; slow: E-selectin) is impacted by PSGL-1 and ESL-1 binding to their selectin partners, as well as CD44, which, in a lipid raft and Bruton tyrosine kinase (BTK)-dependent manner, affects E-selectin–mediated slow rolling. Chemokine-mediated full activation of LFA-1 leads to ICAM-1–dependent firm neutrophil adhesion. This adhesive bond is further strengthened by Mac-1 interactions. Signaling elements and recruitment cues involved in integrin activation include talin-1 and kindlin-3. The subsequent slower crawling toward endothelial access points is primarily controlled by CXCL-1, whereas the transmigration toward the abluminal side is CXCL 2 dependent, followed by another step of abluminal CXCL-1–dependent crawling. Neutrophil transmigration depends on pore size selection and is additionally facilitated by mast cell–derived tumor necrosis factor (TNF). Neutrophils exert their functions within the tissue but are capable of reentering the blood circulation (reverse transmigration) in a Mac-1, neutrophil elastase, LTB-4, JAM-C–dependent pathway, taking on an activated phenotype. Different translational studies and observations have been performed to highlight implications of distinct mechanistic cues in neutrophil recruitment and functionality (upper letters). mAb, monoclonal antibody; MMPs, matrix metalloproteinase; NE, neutrophil elastase; PEU, perivascular extravasation unit; 3D, three dimensional.