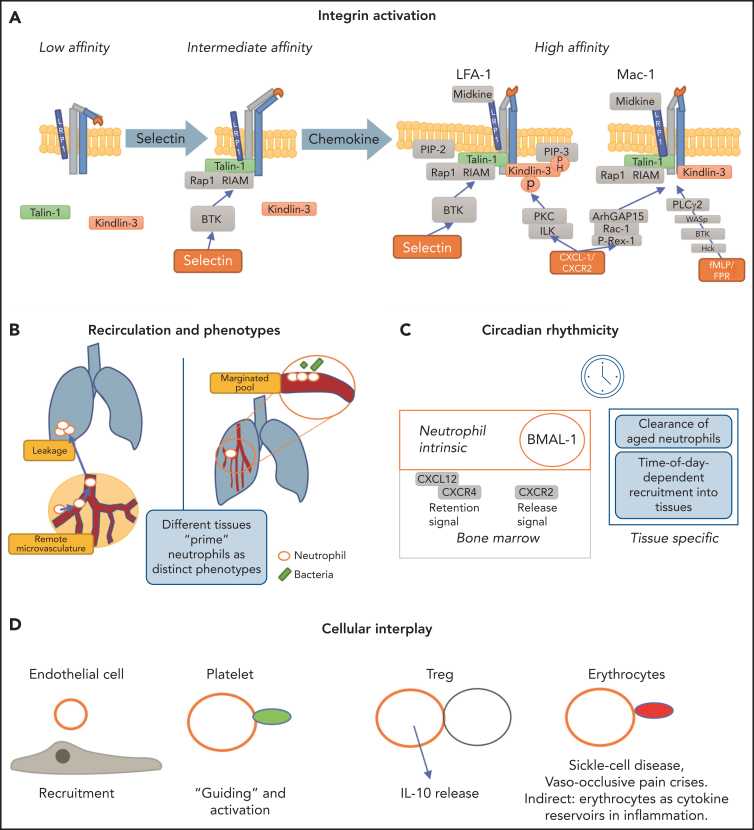
Figure 2.
Regulators of neutrophil trafficking. (A) Integrin activation is distinctly regulated. Selectin engagement results in the intermediate conformation of the integrin, which requires talin-1 binding to the integrin cytoplasmic tail. The high-affinity conformation requires the presence of talin-1 and kindlin-3. Talin-1 binding relies on formation of a complex with Rap1 and RIAM, whereas kindlin-3 is recruited to the integrin prior to induction of the high-affinity conformation and transmits its activation signal toward LFA-1 in an ILK, PKC, and pH-domain–dependent manner. Mechanistic differences between LFA-1 and Mac-1 exist in that Mac-1 activation depends on ArhGAP15 and Bruton tyrosine kinase (BTK). LRP1 can bind via the I domain to Mac-1 and to a lesser extent to LFA-1. (B) Reverse-transmigrated neutrophils can recirculate and impact leakage even at remote organs, such as the lung. The lung-resident marginated pool of neutrophils undergoes fast Abl kinase–dependent phenotypic changes upon LPS challenge to prime the cells for bacterial “hunting.”85 Overall, neutrophils exert effects within different tissues but are equally impacted and modulated by their surroundings. (C) Circadian rhythms dictate leukocyte release, function and clearance, impacting and modulating many subsequent activation and recruitment mechanisms. (D) Neutrophils interact with endothelial cells, which mediates recruitment and activation. Interaction with platelets helps in activation, priming, and recruitment to inflamed or injured tissue but also occurs during thrombus formation. Interaction of neutrophils with regulatory T cells (Treg) evokes interleukin-10 (IL-10) release by neutrophils. Neutrophil-erythrocyte interaction is prominent in sickle cell disease. Additionally, erythrocytes contain a variety of cytokines that can impact neutrophil functionality.