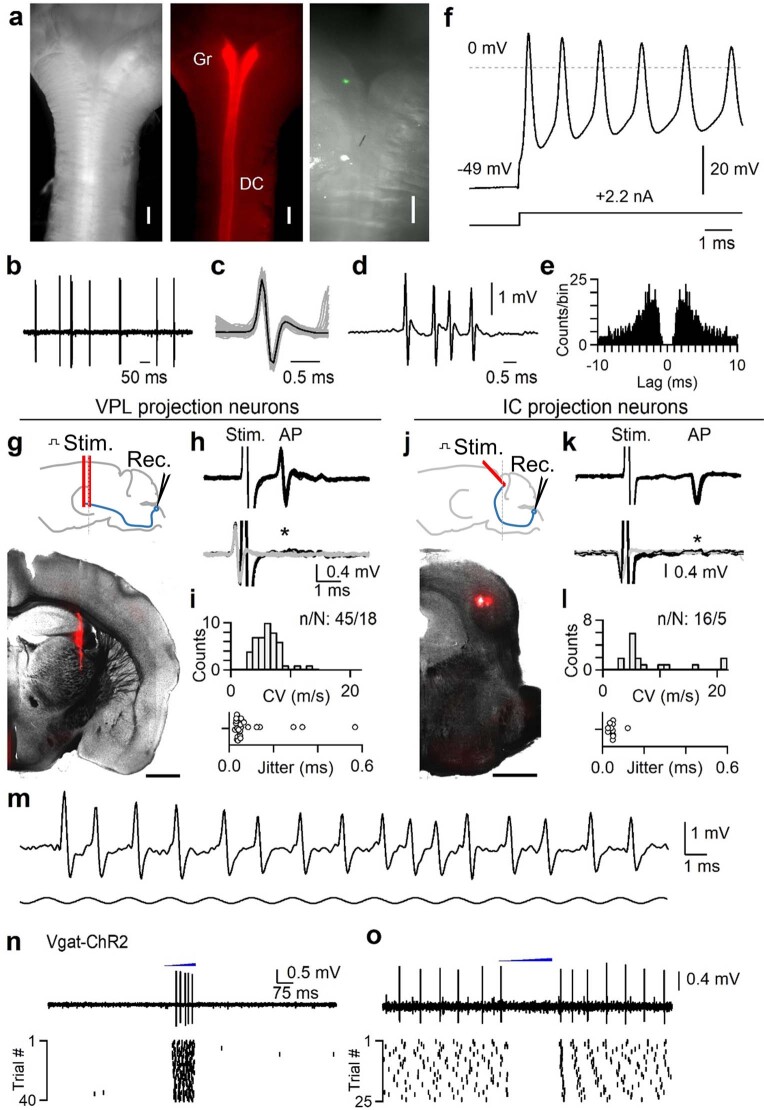
Extended Data Fig. 1. Juxtacellular recording in the DCN, antidromic activation, and optotagging.
Juxtacellular recordings were made from units in the gracile nucleus of the DCN to obtain high signal-to-noise recordings of putative single units. Recordings were obtained in urethane-anesthetized mice. Units in the DCN can fire at very high rates up to 1 kHz and can undergo use-dependent changes in action potential waveforms during bursts55. Many response properties seen in non-anesthetized preparations are present in anesthetized animals, including a wide distribution of receptive field sizes, entrainment to high-frequency vibration, inhibitory surround receptive fields, and persistent responses to sustained stimuli39–41. However, urethane anesthesia will suppress descending input that may modulate the DCN, and may alter polysynaptic circuits that affect DCN activity during different behavioral states. a, Left: Top view showing the dorsal surface of an isolated brainstem with myelinated dorsal columns forming a “Y” shape as they reach the DCN. Middle: AAV1-hSyn-Cre was injected into the lumbar cord of a mouse expressing Rosa26LSL-Acr1-FRed, labeling the dorsal columns (DC) and gracile nucleus (Gr). Right: Zoomed-in view of the gracile nucleus following a recording when the electrode was coated with DiI. The location of the recording site can be seen in green. b, Example of spontaneous firing recorded juxtacellularly in the DCN in vivo. c, Overlaid spikes with single events (gray) and average waveform (black). d, Example high-frequency burst. e, Autocorrelogram for unit in a–d. f, Ex vivo whole-cell recording of a DCN neuron from an intact brainstem of an adult mouse. Recording was performed in near-physiological conditions (35 °C). A current injection was supplied to trigger a brief burst of very high frequency firing. Cells could fire up to 1 kHz bursts with use-dependent changes in action potential waveform as observed in vivo. Three random large-diameter cells were recorded ex vivo in one animal. g, Identification of VPL-projection neurons (VPL-PNs). Top: schematic of preparation, a bipolar electrode was lowered into the VPL. Bottom: Example coronal section (100 µm thickness) showing track of the stimulating electrode that was coated with DiI. Scale bar is 1 mm. Anatomical location of stimulus electrode was verified in two animals. h, Top: A unit in the DCN antidromically activated from the VPL. Overlay of 20 trials shows consistent waveform of the stimulus artifact (stim.) and antidromic action potential (AP). The conduction velocity was calculated from the latency to spike following the stimulus artifact with an estimated distance of 9.8 mm. Bottom: during a collision test, the stimulus was triggered from the spontaneous firing of the unit. Overlay of 20 trials when electrical stimuli were triggered from spontaneous firing (black). In these trials, stimulation failed to evoke an antidromic spike that would be expected at the latency indicated by the asterisk. Waveform of spontaneous firing in which stimuli were not triggered are overlaid in gray. i, Top: Histogram of calculated conduction velocities for units antidromically activated from the VPL. Distance from the DCN to VPL was estimated to be 9.8 mm. Bottom: Jitter (standard deviation) of latency from units antidromically activated from the VPL that passed collision testing. j, Identification of IC-projection neurons (IC-PNs). Top: schematic of preparation, a bipolar electrode was angled at 45° in order to avoid the sinus above the IC. Bottom: Example coronal section (100 µm thickness) showing track of the stimulating electrode that was coated with DiI. Scale bar is 1 mm. Anatomical location of stimulus electrode was verified in two animals. k, Same as in h, but for a unit antidromically activated from the IC. l, Same as in i, but for units activated from the IC. Distance from the DCN to IC was estimated to be 9.4 mm. IC units are those that were classified as vibration-tuned only (see Extended Data Fig. 2). m, Juxtacellular recording of a single DCN unit in vivo showing response to high frequency (500 Hz) vibration of the hindlimb. The unit could entrain to single cycles of the vibration and underwent use-dependent changes in waveform. Vibration begins 6 ms prior to displayed time window. n, Optotagging of inhibitory interneurons in the DCN in a Vgat-ChR2 mouse. A fiber optic was positioned above the DCN (400 µm diameter, 1 mm away). Light from a fiber-coupled LED was ramped for 200–300 ms (470 nm, 1-2 mW/mm2). Optotagged units fired during the ramp. A single trial (top) and raster of all trials (bottom) for an optotagged unit are shown. o, Units that were not optotagged were suppressed by light ramps, or fired rebounds after termination of the ramp. Data shown as n units in N animals (n/N).