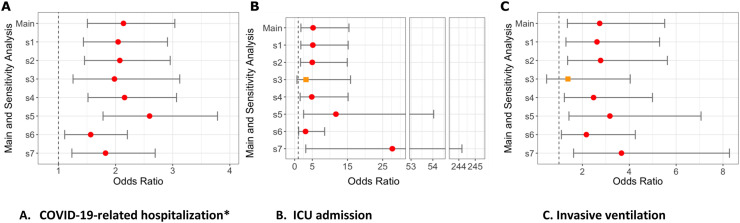
Fig. 3.
Sensitivity analyses (S1-S7) for odds of hospitalization, ICU admission or invasive ventilation with rituximab from US N3C cohort.
legend Figure shows odds ratios and 95% confidence interval for association between rituximab use and A) COVID-19-related Hospitalization; B) ICU admission, C) Invasive ventilation.
New S1, sensitivity analyses 1: Deyo-Charlson overall score (with RA excluded) as 0, 1, 2 vs. 3 or more.
New S2, sensitivity analyses 2: Each Deyo-Charlson comorbidity (with RA excluded).
New S3, sensitivity analyses 3: RA Case definition requires at least 2 ICD codes.
New S4, sensitivity analyses 4: RA Case definition requires >= 1 ICD code + any RA medication.
New S5, sensitivity analyses 5: Referent is Hydroxycholoroquine, nbDMARD variable except HCQ, i.e., Methotrexate, Leflunomide, or Sulfasalazine.
New S6, sensitivity analyses 6: Referent is No DMARD category.
New S7, sensitivity analyses 7: Referent is TNFi-biologic category.
Hospitalized*: adjusted for demographics, weight categories per BMI as normal vs. underweight, overweight, and obese, smoking status, US region, and modified Deyo-Charlson index.
All other outcomes: adjusted for above variables and all COVID-19 treatments.
Circles (red) denote significant outcomes, orange squares denote non-significant outcomes.