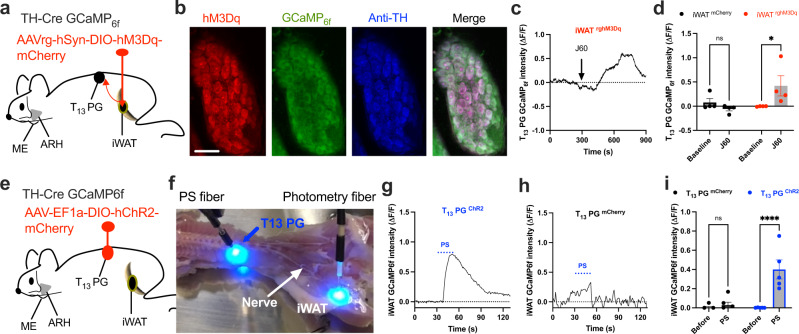
Fig. 4. Functional evidence of T13 PG providing sympathetic inputs to inguinal iWAT.
a Schematic illustration of targeting a retrograde vector carrying Cre-dependent hM3Dq-mCherry to inguinal iWAT in dual TH-Cre GCaMP6f mice. b Sample images showing colocalizations of retrograde traced and hM3Dq-mCherry (red)-transduced TH neurons stained with Alex Fluor 350-conjugated anti-TH antibodies (blue) which also expressed GCaMP6f (green) in T13 PG. c, d Ex vivo photometry monitoring was performed at acute isolated T13 PG from the retrograde traced and hM3Dq-transduced TH-Cre GCaMP6f mice, and chemogenetic stimulation of the hM3Dq-transduced TH neurons with J60 addition to the circulating aCSF increased the intensity of GCaMP6f signals in the T13 PG: c A representative trace of real-time monitoring of T13 PG GCaMP6f signals with J60 addition as pointed; and d Group data of T13 PG GCaMP6f signals in iWAT injected with hM3Dq- or mCherry (n = 4 per group). e–i PS of ChR2-transduced T13 PG excited the sympathetic neural inputs in ex vivo inguinal iWAT: e Illustration of targeting a vector carrying Cre-dependent ChR2 to T13 PG in dual TH-Cre GCaMP6f mice; f Ex vivo intact spinal cord-iWAT tissues with a PS fiber placed on exposed T13 ganglion and a photometry fiber placed on the ACFN entry point in inguinal iWAT in a recording chamber; g–i Representative photometry monitoring of GCaMP6f signals with PS of T13 PG g ChR2- and h mCherry-transduced mice, and i Group data of average GCaMP6f signals from g and h (mCherry, n = 4; ChR2, n = 5). Two-way ANOVA with Sidak post hoc tests; data represent mean ± s.e.m; p = 0.67 (d, mCherry); *p = 0.04 < 0.05 (d, rghM3Dq); p = 0.91 (i, mCherry); ****p < 0.0001 (i, ChR2); n.s. (not significant). Scale bars for b 50 μm. PG, paravertebral ganglia. Arrow in c indicated the addition of J60 in the circulating aCSFs. Blue dotted lines in g and h indicated the PS duration (20 Hz for 1 s; repeated every 4 s for 30 s).