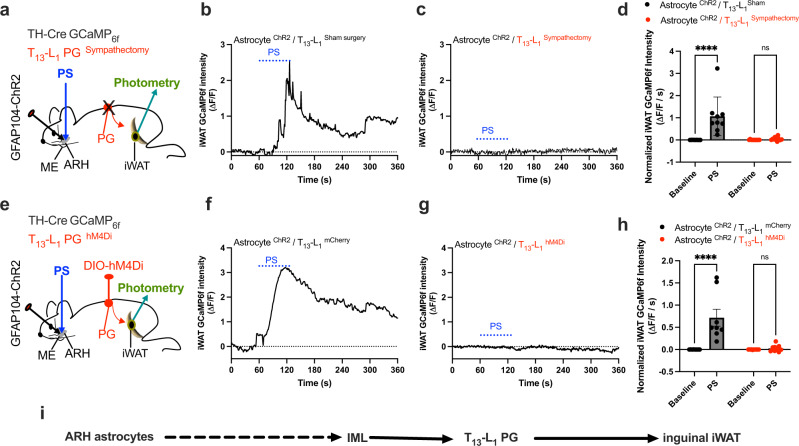
Fig. 5. T13-L1 PG sympathectomy or inhibition blunts astrocyte promoting the sympathetic drive to iWAT.
a Schematic illustration of targeting a vector carrying GFAP104-dependent ChR2 to the ARH and implanting an optic fiber over ARH, and placing a photometry fiber in inguinal iWAT in T13-L1 PG sympathectomy dual TH-Cre GCaMP6f mice. b–d PG sympathectomy blunts astrocyte stimulation-induced excitation of iWAT sympathetic inputs: b, c Representative photometry monitoring of TH-positive nerve GCaMP6f signals with PS of ChR2-transduced astrocytes in the PG b Sham- and c sympathectomy mice respectively, and d Group data of average GCaMP6f signals from b and c (n = 9 per group). e Schematic illustration of targeting a vector carrying Cre-dependent hM4Di to T13-L1 PG in astrocyte ChR2-transduced and ARH-implanted dual TH-Cre GCaMP6f mice. f-h: Inhibition of the PG with J60 via i.p. 30 min prior to performing photometry blunted the PS-induced effects: Representative photometry monitoring of TH-positive nerve GCaMP6f signals with PS of ChR2-transduced astrocytes in the PG f mCherry - and g hM4Di-transduced mice, and h Group data of average GCaMP6f signals from f and g (n = 8 per group). i A hypothetical neural pathway for central astrocyte regulation of iWAT through the PG. Two-way ANOVA with Sidak post hoc tests; data represent mean ± s.e.m.; ****p < 0.0001 (d, AstrocyteChR2/T13-L1Sham); p = 0.99 (d, AstrocyteChR2 /T13-L1Sympathectomy); ****p < 0.0001 (h, AstrocyteChR2/T13-L1mCherry); p = 0.94 (h, AstrocyteChR2/T13-L1hM4Di); n.s. (not significant). Blue dotted lines in b, c, f, and g indicated the PS duration (20 Hz for 1 s; repeated every 4 s for 60 s).