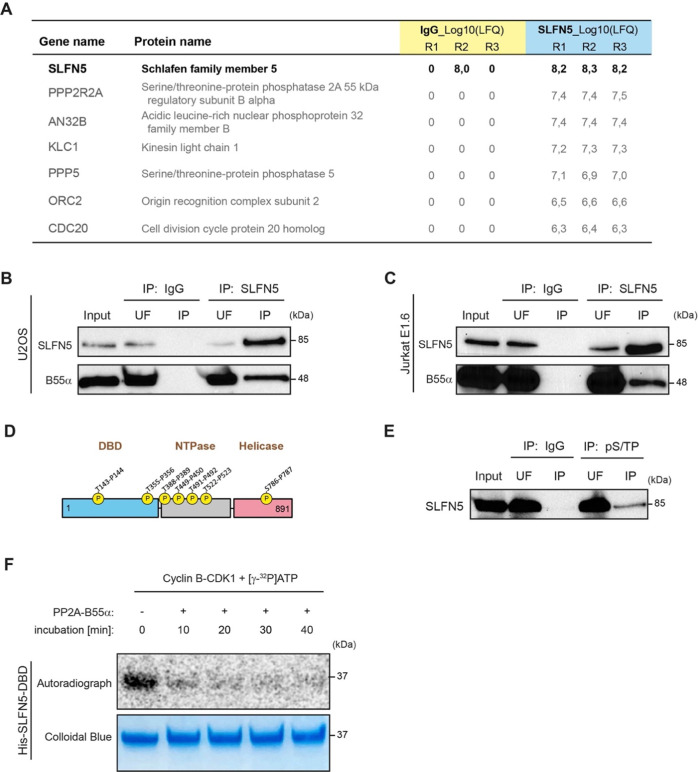
Fig. 2. PP2A-B55α interacts with SLFN5 and dephosphorylates its CDK minimal consensus sequences.
A Table summarizing the strongest interactors identified by mass spectrometry after SLNF5 immunoprecipitation. For each protein, the log10 values of the label-free quantification intensities (LFQ) in the three replicates of the IgG-IP and SLNF5-IP are reported. B, C Validation of SLFN5 - B55α interaction in U2OS and Jurkat E1.6 cells by co-immunoprecipitation followed by Western blotting. #112/5/6 anti-SLFN5 was used to co-immunoprecipitate endogenous SLFN5 and its interactors. Immunoprecipitation with an irrelevant mouse IgG antibody served as a negative control (control IP). B55α recovery was confirmed using an antibody against the regulatory subunit B55α. UF, unbound fraction. The Western blot shows a representative experiment from three independent validations. D SLFN5 contains a cluster of five TP motifs in the DBD and NTPase domain, and single TP (T143-P144) and SP (S786-P787) motifs at the N-terminus and C-terminus, respectively. E SLFN5 is phosphorylated at T/SP minimal CDK consensus sequences. An anti-pT/SP antibody was used for immunoprecipitation in U2OS cells. The membrane was probed with #112/5/6 anti-SLFN5. Immunoprecipitation with an irrelevant mouse IgG antibody served as a negative control. The Western blot shows a representative experiment from three independent experiments. F SLFN5-DBD TP sites are phosphorylated by Cyclin B-CDK1 and dephosphorylated by PP2A-B55α. Timecourse of SLFN5-DBD dephosphorylation. Comassie staining serves as loading control. The Radiograph shows a representative experiment from three independent experiments.