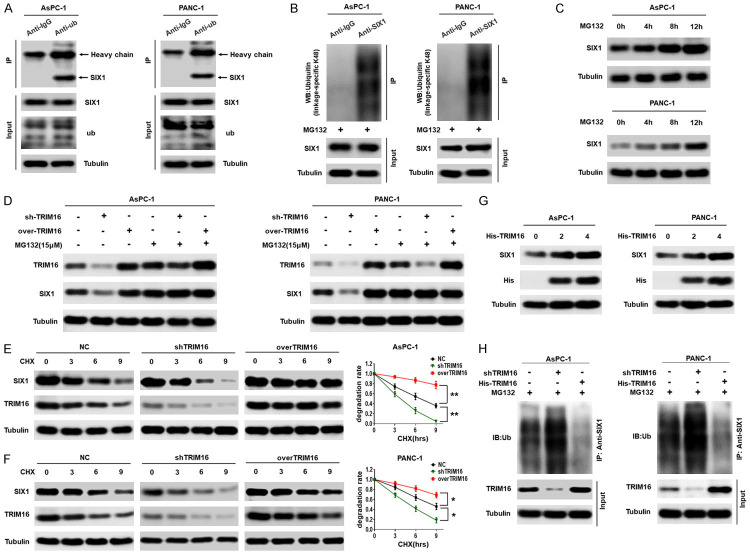
Figure 5.
TRIM16 stabilizes SIX1 protein by inhibiting its ubiquitination. A. Representative western blot showing that Ubiquitin interacts with SIX1 in pancreatic cancer cells. Pancreatic cancer cells were lysed, and an immunoprecipitation assay was performed with anti-IgG or anti-ub antibodies, followed by western blot with the indicated antibodies. B. Ubiquitinated SIX1 in the lysates of AsPC-1 and PANC-1 cells was precipitated with anti-IgG or anti-SIX1 after the cells were treated with MG132 (15 mmol/l) for 12 h, followed by western blot to detect ubiquitin (linkage-specific K48). C. AsPC-1 and PANC-1 cells were treated with MG132 for the indicated times, the levels of SIX1 protein were detected by western blot. D. Transfecting TRIM16-silenced vector or TRIM16-overexpressing vector into AsPC-1 and PANC-1 cells, and using western blot to detect the protein levels of TRIM16 and SIX1 with or without MG132 treatment. E, F. AsPC-1 and PANC-1 cells were stably transfected with the TRIM16-silenced or TRIM16-overexpressing vector. The cells were subjected to cycloheximide (CHX, 20 mmol/L) exposure for the indicated times and the protein level of SIX1 was detected. The degradation rate was converted into a statistical linear graph (*P<0.05, **P<0.01, nonlinear regression, exponential one-phase decay). G. AsPC-1 and PANC-1 cells were transfected with increasing amounts of His-trim16 plasmid. The protein levels of SIX1 were detected with anti-SIX1 antibody. H. AsPC-1 and PANC-1 cells were stably transfected with the TRIM16-silenced or TRIM16-overexpressing vector. The level of ubiquitin-attached SIX1 was precipitated with anti-SIX1 after the cells were treated with MG132 for 12 h, followed by western blot to detect ub.