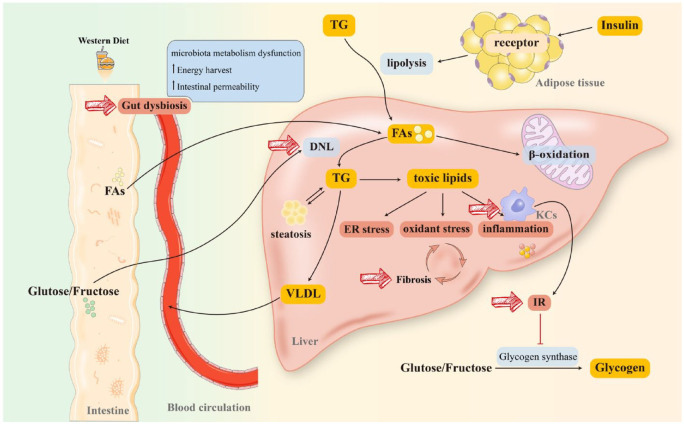
Figure 1.
Pathogenesis and therapeutic targets of NAFLD. Long-term consumption of a WD will substantially reprogram the intestinal microbiome. Furthermore, FAs from diet or lipolysis of TG (stimulated by the combination of insulin and receptor on lipocytes) are transported to the liver through blood circulation. As a critical element in initiating NAFLD, IR has been hypothesized to connect with lipid intake. Moreover, IR activates KCs and impairs glycogen synthase, more dietary glucose and fructose reach the liver, resulting in massive FAs accumulation in the liver. A proportion of FAs participates in β-oxidation in mitochondria and peroxisomes to produce energy, while the rest stimulates DNL to synthesize TG. TG is degraded in two ways: (1) being transported to peripheral organs in the form of VLDL and (2) producing lipotoxic lipids (diacylglycerols, ceramides, lysophosphatidyl choline species, cholesterol) that induce mitochondrial dysfunction, ER stress, and inflammatory responses, promoting fibrosis to exacerbate NAFLD/NASH. Diet-induced gut dysbiosis also increases energy harvest and intestinal permeability, as well as perturbs microbiota metabolism, leading to NAFLD progression and pathogenesis. Together, gut dysbiosis, DNL, IR, KCs activation, and fibrosis are all plausible targets for NAFLD management.
DNL, de novo lipogenesis; ER, endoplasmic reticulum; FAs, fatty acids; IR, insulin resistance; KCs, Kupffer cells; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; TG, triglyceride; VLDL, very low-density lipoprotein; WD, Western Diet.