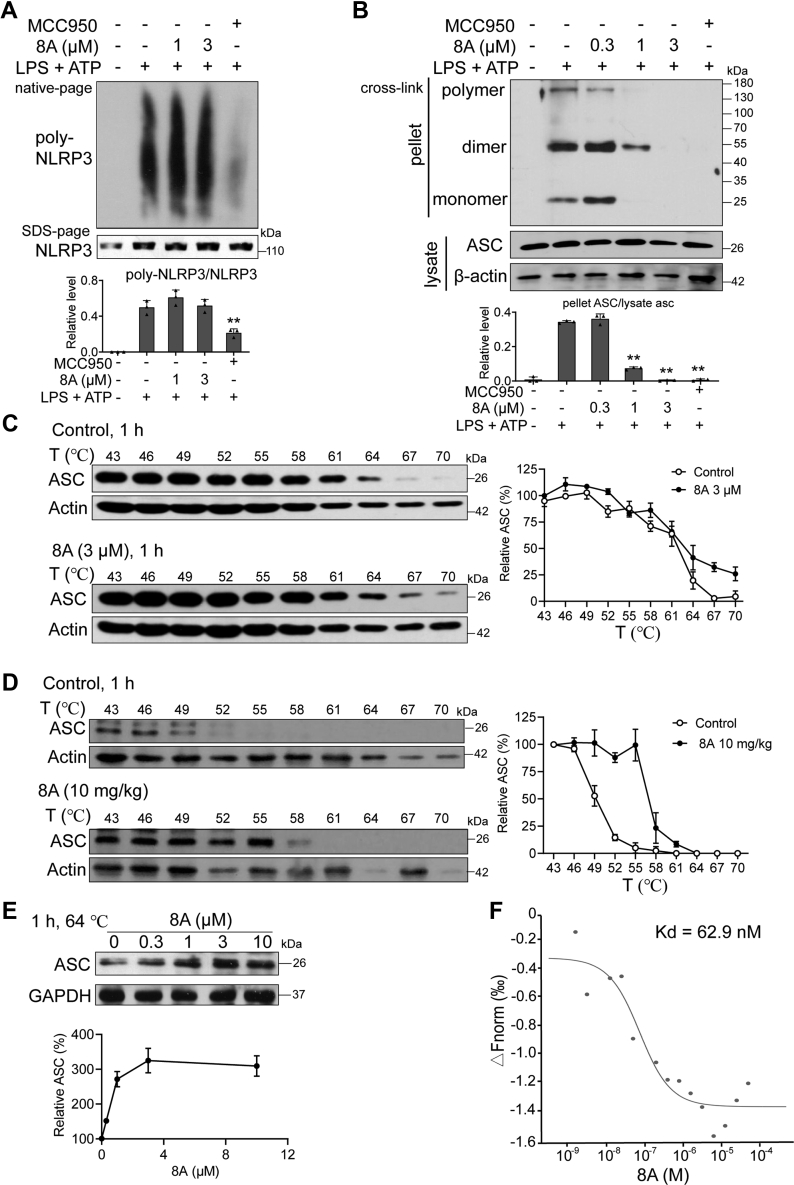
Figure 4.
8A inhibited ASC oligomerization via direct interaction.A and B, LPS-primed BMDM were treated with 8A for 1 h, followed by ATP stimulation for 15 min. A, oligomerization of NLRP3 was analyzed using blue native PAGE. B, oligomerization of ASC in whole lysates was examined using Western blotting. C, BMDMs were incubated with 8A (DMSO as control) for 1 h, and cells were collected and subjected for CETSA. D, mice were i.g. with 10 mg/kg 8A once a day for 3 days. Peritoneal macrophages were collected and subjected to CETSA. E, BMDM cells were incubated with indicated concentrations of 8A for 1 h, and cells were collected subjected for CETSA at 64 °C. F, lysates from 293T cells transfected with ASC-GFP plasmid were used to determine the binding between ASC and 8A. Data were representative of three independent experiments. Results were presented as the mean ± SD of three independent experiments. ∗p < 0.05, ∗∗p < 0.01 versus ATP group or as indicated. ASC, apoptosis-associated speck-like protein containing a caspase activation and recruitment domain; BMDM, bone marrow-derived macrophage; CETSA, cellular thermal shift assay; LPS, lipopolysaccharide; NLRP3, Nod-like receptor family protein 3.