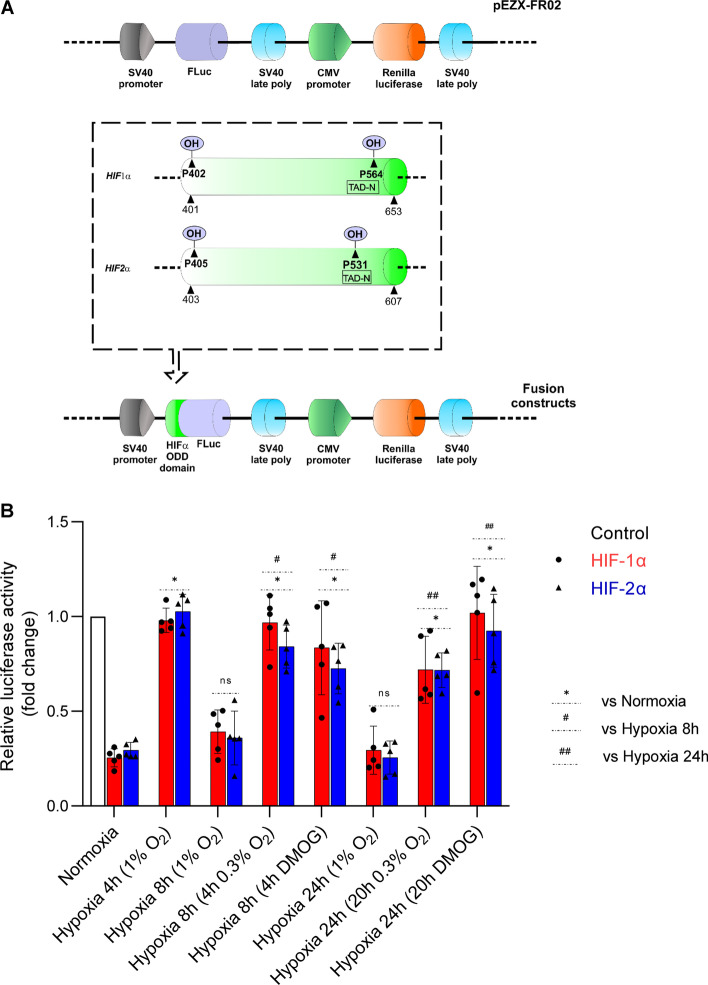
Fig. 4.
Prolyl hydroxylase inactivation in HUVECs influences ODD domains stability during hypoxia. A Schematic presentation of firefly luciferase (FLuc) and renilla luciferase reporter cloning vector pEZX-FR02 (GeneCopoeia) containing the HIF1A human (NM_181054) gene region comprising ODD (amino acids 401–653, including the Prolines 402 and 564 (a PHD substrate) or human EPAS1 (NM_001430) gene region comprising ODD (amino acids 403–607, including the Prolines 404 and 531 (a PHD substrate). These gene regions were fused with firefly luciferase (without the ATG start codon) downstream of the HIF-α. B Hypoxia-related stabilization of the HIF-1α and HIF-2 α ODD reporter. HUVECs following the transfection with the HIF-α-ODD reporter vectors were exposed to hypoxia (1% O2) for indicated time points in the presence or absence of 2.5 mM DMOG, or exposed to 0.3% O2 (both DMOG addition and the exposure to 0.3% O2 were performed after 4 h form the experiments start) and luciferase activity measured with Dual-Luciferase Reporter Assay System. The locations of the proline hydroxyl addition that destabilizes the HIF alpha subunits is illustrated in the panel A box. The firefly luciferase data were normalized to Renilla signal and expressed as a fold change over the control vector (without HIF-α-ODD) in the same experimental conditions. R.L.U. – relative light units. Data represent the mean ± SD of five independent experiments (3 replicates each). The P < 0.05 was considered significant and depicted as follows: * (for pairwise comparison to normoxia), # (for pairwise comparison to hypoxia 8 h), ## (for pairwise comparison to hypoxia 24 h)