Abstract
Genital infection with Chlamydia trachomatis results in both the local recruitment of protective immune responses and an inflammatory infiltrate that may also participate in tubal pathology. As a beginning to understanding the etiology of immune system-mediated tubal pathology, we evaluated the regional recruitment of lymphocyte subsets to different areas of the female genital tract (GT) over the course of a murine infection with the mouse pneumonitis agent of Chlamydia trachomatis (MoPn). Using flow cytometric techniques we found that the CD4 lymphocyte subset was preferentially recruited to the upper GT (oviduct and uterine horn) over the lower GT (cervical-vaginal region) throughout the course of MoPn infection. The influx of CD4 cells also correlated with the expression of endothelial cell adhesion molecules (ECAMs) and in vitro lymphocyte adherence in the upper GT. Interestingly, the expression of ECAMs in the lower GT was not maintained longer than 7 days after infection, even in the presence of viable chlamydiae. Taken together, these data suggest that regulatory mechanisms of lymphocyte recruitment differ between the upper and lower regions of the GT and may influence the clearance of chlamydiae and the development of tubal pathology.
Infection with Chlamydia trachomatis remains the most prevalent type of bacterial sexually transmitted disease within the United States (1). Although the great majority of infections are asymptomatic, a Chlamydia infection predisposes females to the development of pelvic inflammatory disease (PID) and infertility due to scarring fibrosis of the fallopian tubes (42). Thus, understanding the basis for developing the pathologic sequelae associated with chlamydial infections is important for the design of protective vaccines or therapeutic interventions. The mechanisms which mediate these pathologic changes are not clear at present; however, immune system-mediated damage is thought to play a role. For instance, in humans multiple episodes of PID increase the risk of developing tubal occlusion (46) and, in primates, multiple successive infections are linked with the appearance of tubal pathology (33). Conversely, a prolonged or chronic infection also increases the likelihood of PID in humans (42).
Investigations exploring the possible immune system-mediated mechanisms of pathology have been carried out most extensively with mice. Studies using major histocompatibility complex class II (27) or T-cell receptor-β knockout mice revealed that in the absence of a T-cell response, upper genital tract (GT) pathology developed. This finding was also corroborated following the infection of SCID mice (9). Furthermore, the continued presence of inflammatory infiltrates was observed in nude mice that were unable to eradicate chlamydiae from the GT (41). Therefore, while immune system-mediated damage may contribute to tubal pathology following chlamydial genital infection, these data also predict that the lack of a chlamydiacidal T-cell response would prolong infection and expedite the development of pathologic changes.
It has been shown that the appearance of an antichlamydial T-cell response in the local genital mucosa coincides with the clearance of live organisms (7, 18). However, recent evidence indicates that recruitment of the appropriate type of CD4 cell population is necessary for the rapid clearance of chlamydiae and decreased pathology. For instance, the local recruitment of Th1 cells secreting gamma interferon (IFN-γ) has also been shown to be associated with the clearance of chlamydiae (7) from the local genital mucosa. In addition, blocking the production of the Th1-cell-mediated immune response by the administration of anti-interleukin-12 (anti-IL-12) prolonged the course of infection as well as the presence of purulent exudate in the GT (34). Likewise, the infection of IFN-γ knockout mice (9, 34) or IFN-γ receptor −/− mice (20) resulted in a lengthened course of infection and the development of GT pathology. Finally, the generation of a predominant Th2 immune response, which is ineffective at killing Chlamydia, may also contribute to prolonged infection and pathologic changes (47, 50).
Thus, it is clear from recent findings that the recruitment of Th1 CD4 cells to the infected genital mucosa is necessary to clear infection. The genital mucosa normally contains very few immune cells (31, 32). Therefore, a critical component in the immune clearance of chlamydiae is the recruitment of the appropriate lymphocyte subpopulation to the local genital mucosa. We have previously reported that CD4 cells are recruited in increasing numbers to the GT during infection (22). Furthermore, adhesion molecules are transiently expressed in the GT following infection, appearing as early as 3 days, but diminishing 35 to 50 days, after MoPn inoculation. Since adhesion molecule expression on the endothelium is required for tissue emigration of leukocytes (5), the regulated expression of these molecules is likely to govern lymphocyte recruitment to the GT. In addition, the particular endothelial adhesion molecules that mediate tissue extravasation of Th1 CD4 cells appear to differ from those associated with Th2 cells (2). Thus, the inability of Chlamydia-specific Th1 cells to reach the upper regions of the GT and to be retained at that site may also prolong infection and contribute to the development of upper tract pathology. In this study, we have explored the regulation of CD4 cell recruitment to different regions of the GT during MoPn infection.
(This study was presented in part at the 9th International Symposium on Human Chlamydial Infection, Napa Valley, Calif., 21 to 26 June 1998.)
MATERIALS AND METHODS
Antibodies.
Hybridomas secreting rat anti-CD4 (TIB 207, immunoglobulin G2b [IgG2b]), anti-B220 (TIB 146, IgM), anti-F4/80 (HB-198, IgG2b), and anti-intercellular adhesion molecule-1 (anti-ICAM-1) (CRL 1878, IgG2b) were purchased from the American Type Culture Collection (Manassas, Va.). The following rat monoclonal antibodies were purchased from PharMingen (San Diego, Calif.): anti-CD11b (clone M1/70, IgG2b), anti-CD8α (53-6.7, IgG2a), anti-vascular cell adhesion molecule-1 (anti-VCAM-1) (429, IgG2a), and anti-CD49d (R1-2, IgG2b). A rabbit polyclonal antibody against fibronectin was purchased from Sigma (St. Louis, Mo.). A rat monoclonal antibody directed against mucosal addressin cell adhesion molecule-1 (MAdCAM-1) (MECA-367) was provided by Phil Streeter (Monsanto Corp., St. Louis, Mo.). An IgG2bκ rat myeloma protein (IR863) (14), normal rabbit serum, and rat monoclonal IgG2a (R35-95; PharMingen) were used as control antibodies.
Infection.
Female BALB/c mice, 4 to 6 weeks old, were purchased from Harlan Sprague-Dawley (Indianapolis, Ind.) and were housed according to the American Association of Accreditation of Laboratory Animal Care guidelines. All mice were first injected subcutaneously with 2.5 mg of DEPO-PROVERA (Upjohn, Kalamazoo, Mich.) in 100 μl of sterile phosphate-buffered saline (PBS). DEPO-PROVERA drives mice into a state of anestrus thus eliminating the variability in the rate and severity of infection due to the estrous cycle (37). Seven days later, while under sodium pentobarbital anesthesia, all mice were inoculated intravaginally with 107 inclusion-forming units (IFU) (50% infective dose = 2.5 × 103 IFU) of the mouse pneumonitis agent of C. trachomatis (MoPn) grown in McCoy cells. Infection was monitored every 3 days after inoculation by obtaining cervico-vaginal swabs (Dacroswab type 1; Spectrum Labs, Houston, Tex.). The swabs were stored at −70°C in sucrose-phosphate buffer until analyzed.
Isolation of chlamydiae from cervico-vaginal swabs and tissue homogenates.
Swabs were prepared as previously described (23). Individual wells of McCoy cell monolayers in 96-well plates were inoculated with 200 μl of the solution described above or homogenized GT tissue (11), followed by centrifugation at 1,900 × g for 1 h. The plates were incubated for 2 h at 37°C. At this time, the isolation solutions were removed, fresh cycloheximide medium was added, and the plates were incubated for an additional 32 h. The cultures were then fixed with methanol. MoPn inclusions were identified by the addition of anti-MoPn immune sera and anti-mouse IgG conjugated to fluorescein isothiocyanate (ICN Immunobiologicals, Irvine, Calif.). The inclusion bodies within 20 fields (×40) were counted under a fluorescence microscope, and numbers of IFU per milliliter were calculated.
Isolation of lymphoid cells.
Whole GTs were harvested and separated into the following anatomical segments; cervical-vaginal (CV) region, uterine horns (UH), and oviducts (OD). Single cell suspensions were prepared from pooled tissues (five mice each) of like segments that were minced with scissors and subjected to collagenase digestion (type I; 5 mg/ml; Sigma) for 45 min at 37°C. Single cell suspensions were prepared by expressing the digests through a 70-μm-pore-size nylon mesh in Dulbecco's modified Eagle's medium (DMEM) (Gibco BRL, Gaithersburg, Md.).
Flow cytometry.
Single cell suspensions (2 × 105 to 4 × 105 cells) were stained in DMEM containing 1% bovine serum albumin (BSA; Sigma) and 0.1% sodium azide (staining buffer) using the microplate method as previously described (22). Isolated cells were first incubated with rat anti-mouse cell surface markers, (see “Antibodies”) for 25 min on ice and then washed twice with DMEM containing 10% BSA. The cells were then resuspended in a goat anti-rat IgG-conjugated fluorescein isothiocyanate (BioSource International, Camarillo, Calif.) at a concentration of 20 μg/ml with 10% autologous mouse serum for 25 min on ice. Following the washing step described above, the cells were fixed in PBS containing 1% paraformaldehyde and kept at 4°C until analyzed.
Flow cytometry was performed on a fluorescence-activated cell sorting analyzer equipped with a 488-nm argon laser and Lysys II software (FACScan; Becton Dickenson). The instrument was calibrated with beads (CaliBRITE; Becton Dickenson) using AutoCOMP software, and the same settings were used throughout the study. Dead cells were excluded on the basis of forward-angle and 90° light scatter, and 10,000 gated cells were analyzed for each sample.
Immunohistochemistry.
The lower GT including the vagina and cervix was removed, and a longitudinal incision was made. The resulting tissue was placed cut side down in OCT freezing media (Fisher Scientific, Pittsburgh, Pa.) to prepare frozen blocks as previously described (22). The upper GT, which comprised the UH, OD, and ovaries, was also submerged in OCT freezing media. The sagittal frozen sections were fixed in acetone, washed in PBS, and incubated in methanol-H202 for 30 min to quench endogenous peroxidase activity. Tissue biotin sites were blocked by the addition of avidin followed by biotin. After a tissue-blocking step with goat serum, the primary antibodies were incubated on the tissue section for 45 min at room temperature in a humidified chamber and then washed. A goat anti-rat or rabbit IgG F(ab′)2 antibody conjugated to biotin at 14 μg/ml (BioSource International) and then streptavidin conjugated to horseradish peroxidase (Zymed, San Francisco, Calif.) were added next, and the tissue section was incubated for 45 min. The bound enzyme was visualized with the ImmunoPure metal-enhanced DAB substrate kit (Pierce, Rockford, Ill.) and preserved with crystal mount (Fisher Scientific). The positive-staining venules on the entire section were counted, and the results were expressed as the number per square millimeter of tissue. Photographs were generated by scanning the microscope slides with a color video camera (Sony Electronics, Inc., San Jose, Calif.) and Pax-it! software (Midwest Information Systems, Inc., Franklin Park, Ill.).
Adhesion assay.
The adhesion assay used was a modification of that described by Butcher et al. (6). Briefly, tissue sections of lower and upper GTs (see previous section) were cut as described above and used within 2 h. Mesenteric lymph node (MLN) cells, harvested 7 days after MoPn infection, were added (106 to 2 × 106 cells) to sections in Hanks' balanced salt solution with 1% BSA and incubated at room temperature for 35 min on a rotator. For blocking studies, antibodies were incubated on tissue sections or with MLN cells (25 μg/ml) for 30 min and then the tissue sections were washed prior to performing the adhesion assay. The slides were washed and fixed with 3% paraformaldehyde. The number of adherent cells on 15 random venules was determined for each slide.
Statistics.
Statistical differences in the number of leukocyte subsets per 106 total cells were tested using two-way analysis of variance (ANOVA) and Tukey's post-hoc test. Statistical differences in the number of venules per square millimeter for each GT region were determined using a nonparametric, one-way ANOVA and Dunn's post-hoc test for use with groups containing unequal numbers of values. Statistical differences in the level of infection among the three GT regions (log10 transformation) were determined by two-way ANOVA. The Spearman rank order correlation test was used to determine the strength of association between the numbers of VCAM-1-positive venules and CD4 cells in the upper (OD plus UH) and lower (CV) regions of the GT. This correlation statistic does not assume that the association between two variables is linear. The effect of blocking antibodies was determined using a one-way ANOVA and Dunn's or Dunnett's post-hoc test for use with groups containing unequal or equal numbers of values, respectively. The above statistical tests were suggested by and performed using SigmaStat software based on the distribution of the data (normal or nonparametric) and sample size (Jandel Scientific, San Rafael, Calif.).
RESULTS
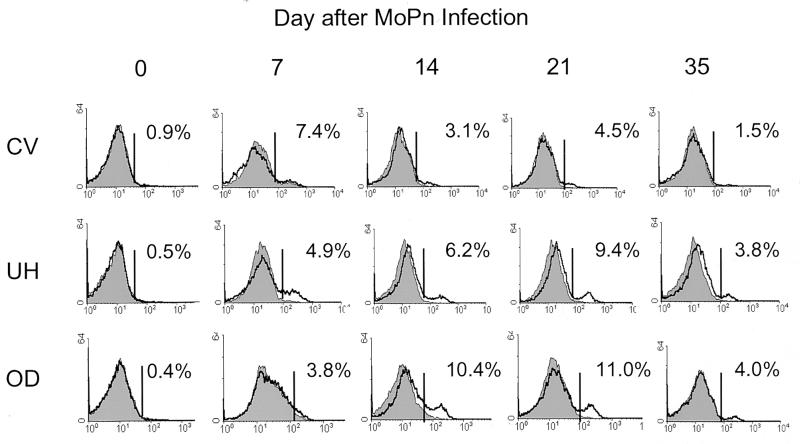
The detrimental effects of chlamydial infection within fallopian tubes manifest themselves as scarring fibrosis and may result from the local release of immune products during a prolonged infection. Many factors could contribute to a protracted course of infection including the production of an ineffective antichlamydial immune response. Alternatively, recruitment of antichlamydial CD4 cells to infected fallopian tubes may be compromised. Therefore we undertook to measure the recruitment of CD4 cells to three different regions of the GT during infection with the mouse pneumonitis agent of C. trachomatis (MoPn). At various time points throughout the course of MoPn infection, the OD, UH, and GT region containing the cervix and vagina were harvested. The numbers of CD4 cells in the OD, UH, and CV regions were determined by flow cytometry on pooled samples of like tissues. As shown in Fig. 1, the percentages of CD4 cells (unshaded histograms) in mice were low to zero prior to infection for all regions of the GT. However, increasing proportions of CD4 cells were detected in the UH and OD by 7 to 14 days after infection, with peak percentages observed at 21 days. Consistent with previous reports (22), the presence of CD4 cells in the genital mucosa was transient and CD4 cells were near preinfection levels 7 weeks after vaginal inoculation (data not shown). In contrast, few CD4 cells were visualized at any time point following MoPn vaginal inoculation in the CV region.
FIG. 1.
CD4 lymphocyte recruitment to different regions of the GT during infection with MoPn. GTs were harvested on various days throughout the course of a MoPn infection. They were dissected into three regions: the CV region, the UH, and the OD. Pooled tissue from five mice were stained for anti-CD4 and analyzed by flow cytometry. The percentage of CD4 cells present in each pool (unshaded histogram) after subtracting the value for the irrelevant control antibody (shaded histogram) is shown.
A comparison of the numbers of CD4 cells appearing over the infection course revealed that not only did the number of CD4 cells in the GT increase over time but also significantly greater numbers of CD4 cells were detected in the UH and OD than in the CV region (P < 0.001; Table 1). This increase was even more profound when the total numbers of GT cells per region were considered. Routinely we found that approximately 5- to 22-fold more total GT cells were isolated from the OD and UH regions than from the CV region throughout the course of infection. Therefore, the absolute numbers of CD4 cells in the UH and OD were greater than that in the CV region. Although CD4 cells first appeared within the infected genital mucosa 7 days after inoculation, significant increases were not noted until 14 to 21 days in the OD and UH, while no significant increases in the number of CD4 cells were detected in the CV region (Table 1). Thus, these results revealed that CD4 cells were preferentially recruited to the upper regions of the GT (OD and UH) over the lower region (CV) during Chlamydia infection.
TABLE 1.
Leukocyte recruitment to different regions of the GT throughout the course of MoPn infection
| Cell type and region | Leukocyte recruitmenta on day:
|
|||
|---|---|---|---|---|
| 0 | 7 | 14 | 21 | |
| CD4b | ||||
| CV | 3.1 (0.1) | 11.9 (7.1) | 26.1 (6.4) | 5.4 (2.1) |
| UH | 6.7 (4.7) | 25.1 (1.4) | 30.3 (2.1) | 48.2 (8.6)c |
| OD | 0.0 | 18.5 (1.9) | 51.8 (2.7)c | 52.5 (12.7)c |
| CD8 | ||||
| CV | 0.0 | 5.2 (7.5) | 13.5 (3.8) | 2.0 (0.9) |
| UH | 0.0 | 3.7 (15.0) | 15.6 (3.8) | 14.9 (0.8) |
| OD | 0.0 | 0.0 | 24.0 (1.9) | 16.2 (4.5) |
| Neutrophil/macrophaged | ||||
| CV | 5.4 (0.3) | 34.9 (9.2) | 54.9 (6.0) | 16.5 (8.1) |
| UH | 11.9 (8.2) | 26.9 (9.0) | 38.2 (6.5) | 30.1 (1.4) |
| OD | 77.4 (70.9) | 153.2 (107.8) | 136.4 (9.9) | 45.5 (8.9) |
| Macrophage | ||||
| CV | 3.5 (0.9) | 1.7 (6.0) | 19.0 (10.1) | 3.1 (0.9) |
| M | 3.4 (2.0) | 3.0 (12.0) | 9.5 (3.7) | 13.3 (4.5) |
| OD | 0.0 | 13.3 (18.7) | 3.8 (4.9) | 10.3 (7.1) |
Values are the means (± standard errors of the means) of two independent pools of GT tissue. Data are expressed as the numbers of CD4-, CD8-, CD11b-, or F4/80-positive cells (in thousands) per 106 total cells recovered from collagenase-digested GTs.
There was a significant increase in CD4 cells in the UH and OD regions compared to the CV region; P < 0.001 by ANOVA.
There was a significant increase in cell numbers compared to day 0 values; P < 0.05 by Tukey's post-hoc test.
There was a significant increase in the number of cells on day 14 compared to day 0; P = 0.037 by ANOVA.
Due to the ability of antichlamydial CD4 cells to mediate protection, we initially focused our efforts on examining this population in the GT. However, CD4 cells are not the only cell population recruited to the GT during MoPn infection (22, 26, 41). Accordingly, we also determined the number of neutrophils, macrophages, CD8, and B cells that appeared in these regions over the course of MoPn infection. As shown in Table 1, cells expressing CD11b, primarily granulocytes and monocytes/macrophages, were recruited in significantly greater numbers to the GT following MoPn inoculation. Unlike what was found for CD4 cells, no preferential recruitment of CD11b-expressing cells to any particular region of the GT was observed. We also noted that a greater number, but not a significantly increased number, of CD8 cells were recruited following infection. Likewise, a trend toward increased recruitment of CD8 cells in the upper regions of the GT was noted (Table 1). Finally, we did not observe any significant increase in the number of B cells (B220-positive cells; data not shown) during the course of infection. These data suggest that recruitment of CD4 cells to the UH and OD is regulated differently from recruitment to the CV region.
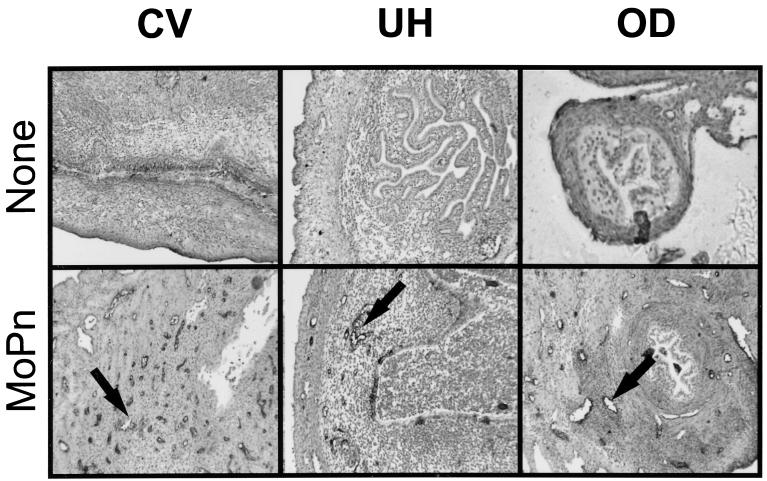
The extravasation of cells into tissue sites depends on the expression of adhesion molecules on endothelial cells lining venules (4). As a beginning to understanding the regulation of CD4 lymphocyte recruitment to various regions of the GT during chlamydial infection, we examined adhesion molecule expression in different areas of the GT. Using immunohistochemical techniques, we first stained the three GT regions with an anti-VCAM-1 monoclonal antibody. As seen in Fig. 2, VCAM-1 expression on the endothelium was absent from all regions of the GT prior to infection (top panels). As expected based on previous studies, shortly following infection, VCAM-1 expression was induced on endothelial venules in the CV region (Fig. 2, bottom left panel). In addition, VCAM-1-expressing venules were prominent both in the UH and the OD, but at time points later in the infection (Fig. 2, bottom center and bottom right panels, respectively). Thus, adhesion molecule expression was observed in all regions of the GT but differences in the kinetic pattern of expression among the three regions appeared to occur over the course of infection.
FIG. 2.
Expression of VCAM-1 in different regions of the GT following MoPn infection. Frozen sections of GTs prepared from uninfected (top panels) and infected mice (bottom panels) were stained with an anti-VCAM-1 monoclonal antibody and visualized using immunoperoxidase histochemistry. Venules staining positive for VCAM-1 can be seen in the CV region 7 days after infection (arrow in bottom left panel), in the UH on day 14 (arrow in bottom middle panel), and in the OD by day 21 (arrow in bottom right panel).
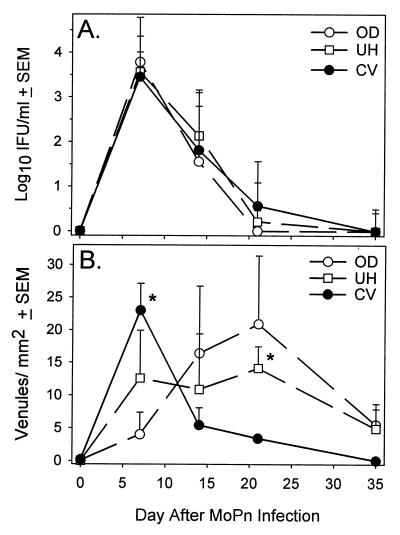
We next quantified the number of VCAM-1-expressing venules throughout the course of infection in order to confirm the relative differences noted in Fig. 2. We also wanted to relate the expression of endothelial cell adhesion molecules (ECAMs) to the levels of infection in different regions of the GT. Consistent with the findings of others, viable chlamydiae were detected in all regions of the GT by day 7 (30). Furthermore, all GT regions tested contained similar numbers of organisms over the course of infection with no significant difference among the regions noted (Fig. 3A). However, we did observe two different patterns of VCAM-1 expression. The first pattern was characterized by a rapid increase in the number of VCAM-1-expressing venules in the CV region, which peaked shortly after infection, on day 7, and then rapidly diminished by day 14 (Fig. 3B). The other pattern of expression was a prolonged or gradual increase in the number of VCAM-1-positive venules that peaked on day 21. However, regardless of the region analyzed, the number of VCAM-1-expressing venules returned to near preinfection levels by day 35. We were intrigued by the rapidly diminishing expression of VCAM-1 in the lower region of the genital mucosa during infection. Generally, adhesion molecule expression is induced by lipopolysaccharide (LPS) and inflammatory mediators such as tumor necrosis factor alpha (TNF-α), which are present in the genital mucosa (10). In addition, the columnar epithelial cells lining the endocervix are the primary site of intracellular chlamydial growth, and this region is contained within the CV sample. Furthermore, viable organisms were recovered on day 14 from the CV region, when VCAM-1-expressing venules were few, and from the OD, where numbers of VCAM-1-expressing venules were elevated (Fig. 3B). Thus, these data suggest that factors that could down-regulate the expression of adhesion molecules in the presence of active infection may be present in the lower genital mucosa.
FIG. 3.
Quantitation of VCAM-1-expressing venules and viable chlamydiae in different regions of the GT throughout the course of MoPn infection. (A) The CV, UH, and OD regions from individual mice were homogenized and cultured for the isolation of chlamydiae. Each data point represents the mean ± standard error of the mean (SEM) of six values. (B) Venules expressing VCAM-1 from each GT region of two to six individual mice harvested on various days throughout MoPn infection were counted. Data are expressed as the means ± SEM. ∗, P < 0.05 (CV region on day 7 and UH on day 21) by ANOVA and Dunn's post-hoc test.
In a previous study, we had also identified venules expressing ICAM-1 and MAdCAM-1 in the murine GT during infection with MoPn (22). We performed a similar quantitative study of both of these adhesion molecules. Although we detected fewer MAdCAM-1-expressing venules than VCAM-1-expressing venules in the GT, we noted similar patterns of temporal expression. Peak increases were seen later in the course of infection in the UH and OD regions, while in the CV region the number of venules peaked on day 7 (Table 2). Similarly, the numbers of venules expressing ICAM-1 in the UH and OD were increased on days 14 to 21 after MoPn inoculation. In the CV region, ICAM-1 expression did not change over time and was present in uninfected mice. These data confirmed that the temporal pattern of ECAM expression seen in the GT over the course of MoPn infection was not unique to VCAM-1.
TABLE 2.
Appearance of MAdCAM-1- and ICAM-1-expressing venules throughout the course of a genital MoPn infection
| Adhesion molecule and region | Mean no. of venules expressing adhesion molecule/mm2 of tissue (± SEM) on day:
|
||||
|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | 35 | |
| MAdCAM-1 | |||||
| CV | 0.04 (0.01) | 3.90 (1.88) | 0.50 (0.40) | 1.14 (0.74) | 0.04 (0.00) |
| UH | 0.02 (0.00) | 0.02 (0.00) | 0.43 (0.36) | 1.42 (0.88) | 0.42 (0.39) |
| OD | 0.09 (0.03) | 0.09 (0.04) | 1.07 (0.55) | 0.43 (0.39) | 0.52 (0.47) |
| ICAM-1 | |||||
| CV | 9.92 (1.25) | 10.85 (1.74) | 6.30 (1.12) | 7.0 (2.87) | 3.94 (1.3) |
| UH | 0.02 (0.00) | 4.89 (2.04) | 9.18 (4.94)a | 15.2 (2.73)a | 1.78 (1.58) |
| OD | 0.09 (0.03) | 0.42 (0.29) | 5.22 (3.35) | 9.10 (4.74) | 1.13 (1.09) |
There was a significant increase in the number of venules compared to day 0 values; P < 0.05 by ANOVA and Dunn's post-hoc test.
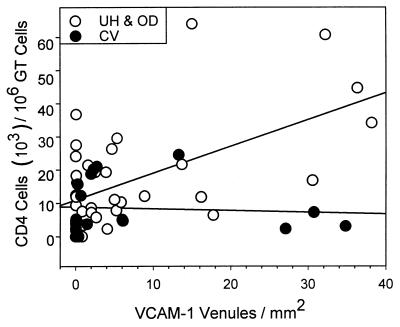
We performed an additional experiment to more directly correlate the expression of adhesion molecules in different areas of the GT with the appearance of CD4 cells. We measured the numbers of CD4 cells from individual mice in different regions of the GT and correlated these parameters to the numbers of venules expressing VCAM-1, as a representative adhesion molecule that was induced upon infection. We found that the number of CD4 cells correlated with the number of venules expressing VCAM-1 in the upper GT (P < 0.04; Spearman rank order correlation test; Fig. 4) but not in the lower tract. Thus, the appearance of VCAM-1-expressing venules correlated with the recruitment of CD4 cells only in the upper tract and may account, at least in part, for the lack of increased numbers of CD4 cells in the CV region during MoPn infection.
FIG. 4.
Correlation of CD4 cells and VCAM-1 expression in the upper versus the lower GT. GTs from individual mice were divided into the upper tract (UH plus OD regions) and the lower tract (CV region) and stained for anti-CD4. The number of CD4 cells was paired with the number of VCAM-1-positive venules per square millimeter for days 0, 7, 14, 21, 35, and 49. Correlation coefficients were obtained using the Spearman rank order test; P < 0.04, n = 37 for the upper GT, and P = 0.156, n = 18 for the lower GT.
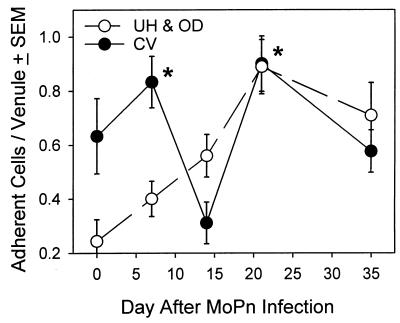
To confirm that the adhesion molecules induced during MoPn infection could mediate functional adhesion, we employed an in vitro adhesion assay (6). We utilized MLN cells, which are known to adhere to MAdCAM-1 as well as VCAM-1 and ICAM-1 (43), from mice harvested 7 days after MoPn vaginal inoculation. We found that the adherence pattern of MLN cells to the genital mucosa was similar to the pattern of CD4 cells recruited throughout the course of MoPn infection. As can be seen in Fig. 5, MLN cell adhesion in the CV region was significantly increased by 7 days after infection but by day 14 the CV region did not support increased adhesion. Moreover, a significant increase in adhesion was not seen in the upper tract until 21 days after infection. Of interest was a second peak of MLN cell adhesion seen in the lower GT region late during the course of infection. We considered fibrin a possible mediator of this increased lymphocyte adhesion, but immunohistochemical staining with an antifibronectin antibody did not reveal increased fibrin deposition compared to that for uninfected mice (data not shown). Furthermore, the number of CD4 cells in the lower GT did not increase at the time when lymphocyte adherence increased (Table 1, day 21). Thus, other factors, such as chemokine release, in addition to ECAM expression must also be contributing to the preferential recruitment of CD4 cells to the upper regions of the infected GT.
FIG. 5.
Lymphocyte adherence on GT sections throughout MoPn infection. MLN cells from mice infected with MoPn were harvested on day 7. Single cell suspensions (2 × 106 cells) were applied to freshly cut frozen GT sections harvested at various days during the course of MoPn infection. Adherent lymphocytes from 10 to 15 venules per section were counted. Cells from multiple sections for each time point from 2 to 4 mice were counted. Each data point represents the mean ± standard error of the mean (SEM) of the number of adherent lymphocytes from 30 to 120 individual venules. ∗, P < 0.05 for the CV region on day 7 and for both the CV region and UH plus OD on day 21 compared to adherence on venules in the UH and OD at day 0, when ECAMs are not expressed (ANOVA and Dunn's post-hoc test).
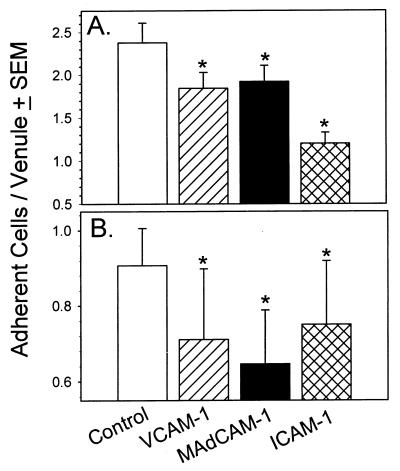
Although VCAM-1, MAdCAM-1, and ICAM-1 were induced in the GT following Chlamydia infection and although lymphocytes could adhere to the infected but not the uninfected GT, we further confirmed that these molecules contributed to lymphocyte adhesion by attempting to block this interaction with specific antibodies. A shown in Fig. 6A, pretreatment of lower-GT tissues with VCAM-1, MAdCAM-1, or ICAM-1 could significantly reduce the adherence of MLN cells compared to that for tissues incubated with control antibody. However, blocking lymphocyte interactions with ICAM-1 resulted in a more complete abrogation of adhesion. Similarly, adhesion in the upper GT was diminished to a similar degree by pretreatment of the tissue with antibodies against all three adhesion molecules (Fig. 6B). Since a low level of fibronectin was found in the GT, we also examined the adhesion of lymphocytes to fibrin by blocking the fibronectin-binding epitope. However, no significant decrease in adhesion was noted (data not shown). Taken together, these data indicate that recruitment of CD4 cells to the Chlamydia-infected genital mucosa is mediated through adhesion interactions with VCAM-1, MAdCAM-1, and ICAM-1 in both the upper and lower regions of the GT. Furthermore, the kinetics of adhesion molecule expression corresponded to the numbers of CD4 cells found after MoPn infection within the different regions of the GT, suggesting a functional role for these molecules in the recruitment of protective CD4 cells.
FIG. 6.
Blocking of lymphocyte adherence in the upper and lower regions of the GT. MLN cells from mice infected with MoPn were harvested on day 7. Monoclonal antibodies against murine VCAM-1, ICAM-1, MAdCAM-1, and an isotype-matched control antibody were incubated on freshly cut GT frozen sections for 30 min at room temperature. Single cell suspensions (2 × 106 cells) were applied to freshly cut frozen GT sections from the CV region on day 7 (A) or the UH plus OD regions on day 14 (B). Adherent lymphocytes from 10 to 15 venules per section were counted. Cells from multiple sections for each time point were counted. Each data point represents the mean ± standard error of the mean (SEM) of the number of adherent lymphocytes from 90 to 139 individual venules. ∗, P < 0.05 by ANOVA and Dunnett's (A) and Dunn's (B) post-hoc test.
DISCUSSION
The expression of adhesion molecules on venules within tissues is necessary for the extravasation of lymphocytes into tissue sites. Therefore, the kinetics and type of adhesion molecules present are factors that regulate the types of leukocytes recruited to tissues during inflammation. We have shown here that the majority of CD4 cells were recruited to the upper GT (UH and OD regions), but not to the lower GT (CV region), in response to MoPn vaginal inoculation. We also observed that the appearance of CD4 cells correlated with the expression of VCAM-1 in the upper tract but not in the lower GT tract. These results indicate that the appearance of VCAM-1 or other adhesion molecules in the upper GT facilitated the recruitment of an increased number of CD4 cells to this site during infection. This was functionally confirmed by the finding that lymphocyte adhesion was not increased until 21 days after infection in the upper tract but was transiently elevated on day 7 in the lower GT. Finally, this adhesion could be blocked by incubating the GT sections with specific antibodies, suggesting the likely participation of these molecules in vivo.
Our studies indicate that a noninfected GT is quiescent with respect to adhesion molecule expression. Therefore, the induction and maintenance of these molecules must be important for cells to access various tissue sites. Unexpectedly, we observed a rapid decrease in VCAM-1 expression in the lower GT but not in the upper tract, suggesting that the regulation of ECAMs differed between the upper and lower regions of the GT. In addition, this decrease in ECAM expression in the lower tract was more remarkable since inflammatory cytokines are known to induce the expression of adhesion molecules (4). In fact, the level of TNF-α in GT secretions was found to increase from days 5 to 8 after MoPn inoculation but then to diminish by day 10 (10). Furthermore, not only was the expression of VCAM-1, MAdCAM-1, and ICAM-1 decreased but also the adhesive ability of the endothelium in the lower GT was significantly diminished 14 days after inoculation with MoPn. Thus, the inflammatory response in the lower GT may possibly be down-regulated by an as yet unknown mechanism.
The induction of the ECAMs (E-selectin, VCAM-1, and ICAM-1) is regulated through the transcriptional activation of the associated genes genes via nuclear transcription factor-κB (NF-κB) after its release from the cytoplasmic inhibitor of NF-κB (IκB) by proteolytic degradation of IκB (39). Pharmacologic agents that inhibit the degradation of IκB have been shown to interfere with the expression of ECAMs in vitro (8). Nitric oxide (NO) has also been reported to limit the expression of VCAM-1 as well as other ECAMs by roughly 50% in the presence of inflammatory cytokines (12). This effect also appeared to be mediated by inhibiting NF-κB activation and was reversed in the presence l-N-monomethyl-arginine. Interestingly, inducible NO synthase knockout mice had a shortened course of infection with MoPn compared to wild-type control mice (35). In addition, IL-10 has also been shown to diminish ICAM-1 expression by targeting NF-κB regulation (40), and this cytokine is present in the GT during MoPn infection (45). Thus, many factors that could suppress as well as enhance ECAM expression are present in the lower GT. Since the response of endothelial cells to inflammatory stimuli differs depending on the tissue (16), further studies of the GT endothelium are needed in order to determine which factors are involved in maintaining this delicate balance.
Another possibility for the diminished expression of ECAM in the lower GT region may not be active down-regulation or inhibition of ECAM at that site but rather the lack of continued stimulation of the endothelium to maintain adhesion molecule expression. For instance, leukocytes themselves appear to stimulate endothelial cells to up-regulate expression of ECAMs. This was shown to be mediated through the release of inflammatory mediators such as TNF-α. Horie et al. showed that the TNF-α induced up-regulation of ICAM-1 and that VCAM-1 was blunted in SCID mice (17). Adoptive transfer experiments revealed that only a T-cell-enriched population could reconstitute ECAM expression. This study also supported the finding that TNFRp55−/− mice were unable to express VCAM-1 in response to TNF-α administration (29). Likewise, the increased production of TNF-α seen in the GT soon after MoPn inoculation (11) most likely participates in the expression of ECAMs. TNF-α primarily utilizes the NF-κB pathway to induce expression of ECAMs, but other pathways for up-regulating the expression of VCAM-1 exist (25). Recently, the cross-linking of ICAM-1 on the surfaces of human umbilical vein endothelial cells (HUVECs) was shown to induce VCAM-1 but not E-selectin through a pathway that is independent of NF-κB (25). Finally, CD40L has also been shown to mediate the induction of ECAMs on HUVECs (21). Thus, activated T lymphocytes recruited to the upper GT may be responsible for maintaining the expression of ECAM at that site both through cell contact and through the release of soluble mediators. Concurrently, the lack of CD4 lymphocyte recruitment to the lower GT may be due to the inability to maintain ECAM expression.
Other subsets of leukocytes are also recruited to GT during infection (Table 1) and may participate in the extended expression of ECAMs in the upper GT. Stagg et al. recently associated the increased recruitment of an antigen-presenting cell (APC) population to the GT of BALB/c mice with more-efficient clearing of chlamydiae compared to that for C3H mice (41). The authors suggested that the presence of these APCs in the local genital mucosa boosted the antichlamydial immune response. One could postulate that local T-cell activation may be necessary to provide a stimulus to endothelial cells for the continued recruitment of lymphocytes, as was suggested by Nakabayashi and colleagues (28). In that report, the absence of major histocompatibility complex class II expression abolished the influx of lymphocytes to the salivary glands of transforming growth factor β knockout mice as well as VCAM-1 expression on the local endothelium. We also observed increased recruitment of a CD11b-positive population, which most likely contains neutrophils and monocytes that could potentially act as APCs and maintain antichlamydial T-cell activation locally.
Although the lack of ECAM expression by T lymphocytes may, in part, explain the selective recruitment of CD4 cells to the upper GT, it does not exclude a mechanism for the damping of immune responses in the lower GT. The lower GT, like the intestinal tract, is a mucosal surface that is in continual contact with potential pathogens and other commensal organisms. Continued exposure to endotoxin or inflammatory cytokines may induce excessive endothelial cell activation. To compensate for this, others have shown that human intestinal microvascular cells display an abbreviated response to LPS, as measured by the length of time ECAMs were expressed following exposure, compared to HUVECs (15). Our findings suggest that the endothelium in the lower GT is hyporesponsive to LPS with respect to adhesion molecule expression. On the other hand, it is also possible that the endothelia in the lower and upper GTs respond equally to an inflammatory stimulus but that other tissue cells secrete modulatory molecules that could act on the endothelium or other cells such as lymphocytes. For instance, intestinal epithelial cells have been shown to inhibit intraepithelial T-cell responses (49). As for the genital mucosa, epithelial cells from various regions of the GT differ in their responses to chlamydial infection. Recently, Wyrick et al. reported that polarized endometrial epithelial cells did not produce TNF-α, IL-1, or IL-8 following infection with Chlamydia (48). In contrast, these authors, as well as Rasmussen and colleagues (38), found that endocervical epithelial cells did produce these cytokines following infection.
Transforming growth factor β is another cytokine that displays immunosuppressive properties and may also neutralize an inflammatory response. Darville et al. reported peak levels in GT secretions 7 days after infection, and these levels diminished to baseline throughout the remainder of the infection course (10). This finding correlates with our evidence for a temporary suppression of the inflammatory response in the lower GT. In Fig. 5, lymphocyte adhesion was increased on day 7 after infection, diminished temporarily on day 14, and then reacquired the ability to again mediate adhesion on day 21. In addition, the expression of MAdCAM-1 followed a similar pattern, with peaks observed on days 7 and 21 (Table 2). Likewise, this pattern of expression was also noted at the mRNA level using reverse transcription-PCR (data not shown). Furthermore, the increased adhesion seen late in the course of infection in the lower GT was not due to fibrin deposition since immunohistochemical staining did not reveal increased fibronectin and since blocking the interaction of lymphocytes with the fibronectin-binding epitope did not diminish adhesion (data not shown). Taken together, these data suggest the presence of some mechanism that temporarily suppresses ECAM expression and possibly the inflammatory response in the lower GT.
The expression of ECAM and the subsequent recruitment of leukocytes to an infection site are complex and involve many factors such as the types and levels of inflammatory cytokines and chemokines. A possible variable in the recruitment of cells at local sites during infection may be the dose of the organism that first comes in contact with the host mucosal surface. It is conceivable that the local mucosal surface may respond in proportion to the dose of the organism. Although this has not been addressed in vivo, some information has been reported on the basis of in vitro studies. Using chlamydial LPS, Ingalls et al. have shown that monocytes produce an increasing amount of TNF-α through ligation of CD14 (19). It is possible that resident tissue macrophages within the GT may produce TNF-α when exposed to chlamydiae. In addition, Beekhuizen et al. (3) showed that a proportional increase in VCAM-1 expression and leukocyte adhesion occurred in response to increasing numbers of staphylococcal organisms. However, the effect correlated to the number of organisms internalized within endothelial cells. Likewise, for chlamydiae, others have shown that adhesion or internalization alone is not enough to induce the release of inflammatory cytokines and chemokines (38) and that replication of the organism within host cells is a necessary prerequisite. For this study, the numbers of organisms replicating within the various regions of the GT were used as a measure of the infectious burden and were found to be equivalent among the three GT regions over the course of infection (Fig. 3A). This data could also be interpreted as indicating that the dose used in this study may have “overloaded” the capacity of the CV region to respond and may explain the abbreviated expression of ECAM noted in Fig. 3B. In this case, using a lower dose may induce a more sustained inflammatory response in the lower GT. However, Darville has found that TNF-α levels do not significantly change with different infecting doses in the murine model (T. Darville, personal communication). Also, preliminary data from the guinea pig model have shown that the number of CD4 cells recruited to upper or lower regions for the GT did not differ between groups infected with 106 and 107 IFU (R. G. Rank, personal communication). Furthermore, a study using the cat model of Chlamydia psittaci ocular infection also reported no change in the magnitude of the immune response in relation to dose but did report differences in the incidence of infection (44). Since the local control of lymphocyte recruitment depends on a series of factors and since the infecting dose for humans is not known, further studies examining the relationship of the levels of cytokines, chemokines, and leukocyte influx to the infecting dose should aid in our understanding of the local human immune response to C. trachomatis.
Based on our results and other published reports, we propose the following series of events as a possible scenario to explain the preferential recruitment of CD4 cells to the upper but not the lower GT. The infection of local epithelial cells causes the release of inflammatory mediators, including chlamydial LPS, that induces the local release of TNF-α early after infection (11) and up-regulation of ECAMs. As suggested by others, an early influx of monocytes may also participate in the production of TNF-α and further stimulate the up-regulation of adhesion molecules (13). As Chlamydia-specific, activated T cells begin to immigrate into the infected areas, they in turn stimulate endothelial cells through cell contact and/or soluble mediators to maintain ECAMs. In the presence of the antigen, continual T-cell activation may occur through recently immigrated or resident APCs. In addition, locally recruited lymphocytes may induce the secretion of a specific array of chemokines through the activation of endothelial cells, as shown for HUVECs in vitro (26), that could attract Th1 CD4 cells over other lymphocyte subsets. This could then create an amplification loop in the upper GT that would continue to preferentially recruit Th1 CD4 cells in the presence of a local antigen. In contrast, in the lower GT, endothelial cells may differentially respond to inflammatory stimuli by shortened expression of ECAMs. In addition, factors which may inhibit lymphocyte responses and interfere with amplified recruitment may be released, possibly by local epithelial cells. Other leukocytes, such as monocytes, NK cells (45), and neutrophils, do not appear to possess the ability to sustain the amplification recruitment loop. The overall effect would result in the preferential recruitment of Th1 CD4 cells to the upper GT and the eradication of infection. Moreover, the accessibility of the upper GT to leukocyte recruitment may also predispose this site to the consequences of immune system-mediated pathology.
The ability to dampen immune responses in the lower GT, while beneficial to the host may also be of benefit to chlamydiae. One could hypothesize that following the induction of a local inflammatory response to Chlamydia in the endocervix, an abbreviated inflammatory response could potentially result in the delayed clearance of chlamydiae from the lower GT. We noted a consistent delay in the clearance of organisms from the CV region compared to the UH and OD (Fig. 3). Although it was not significant, this trend was observed in each experiment. Interestingly, there is also evidence for this in humans. Kiviat and colleagues performed both culture and direct immunofluorescent staining for Chlamydia in the cervix, endometrium, and both fallopian tubes of each of a group of individuals suspected for PID. They reported that in over 50% of the patients, chlamydiae were detected in the cervix but not upper tract by both direct fluorescent-antibody assay DFA (52%) and culture (66%) (24). A phenomenon such as this, if verified, may enhance chronic infection or persistence in the lower GT. Therefore, the difference in regulatory mechanisms of lymphocyte recruitment between the upper and lower regions of the GT may influence the clearance of chlamydiae and may also play a role in the development of tubal pathology.
ACKNOWLEDGMENTS
We thank Phil R. Streeter for supplying the murine anti-MAdCAM-1 antibody and helpful discussion.
This work was supported by NIH AI-26328.
REFERENCES
- 1.Anonymous. Chlamydia trachomatis genital infections—United States, 1995. Morbid Mortal Weekly Rep. 1997;46:193–198. [PubMed] [Google Scholar]
- 2.Austrup F, Vestweber D, Borges E, Lohning M, Brauer R, Herz U, Renz H, Hallmann R, Scheffold A, Radbruch A, Hamann A. P- and E-selectin mediate recruitment of T helper 1 but not T helper 2 cells into inflamed tissues. Nature. 1997;385:81–83. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- 3.Beekhuizen H, Van de Gevel J S, Olsson B, van Benten I J, Van Furth R. Infection of human vascular endothelial cells with Staphylococcus aureus induces hyperadhesiveness for human monocytes and granulocytes. J Immunol. 1997;158:774–782. [PubMed] [Google Scholar]
- 4.Bevilacqua M P. Endothelial-leukocyte adhesion molecules. Annu Rev Immunol. 1993;11:767–804. doi: 10.1146/annurev.iy.11.040193.004003. [DOI] [PubMed] [Google Scholar]
- 5.Butcher E C. The regulation of lymphocyte traffic. Curr Top Microbiol Immunol. 1986;128:85–122. doi: 10.1007/978-3-642-71272-2_3. [DOI] [PubMed] [Google Scholar]
- 6.Butcher E C, Scollay R G, Weissman I L. Lymphocyte adherence to high endothelial venules: characterization of a modified in vitro assay, and examination of the binding of syngeneic and allogeneic lymphocyte populations. J Immunol. 1979;123:1996–2003. [PubMed] [Google Scholar]
- 7.Cain T K, Rank R G. Local Th1-like responses are induced by intravaginal infection of mice with the mouse pneumonitis biovar of Chlamydia trachomatis. Infect Immun. 1995;63:1784–1789. doi: 10.1128/iai.63.5.1784-1789.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen C C, Rosenbloom C L, Anderson D C, Manning A M. Selective inhibition of E-selectin, vascular cell adhesion molecule-1, and intercellular adhesion molecule-1 expression by inhibitors of I kappa B-alpha phosphorylation. J Immunol. 1995;155:3538–3545. [PubMed] [Google Scholar]
- 9.Cotter T W, Ramsey K H, Miranpuri G S, Poulsen C E, Byrne G I. Dissemination of Chlamydia trachomatis chronic genital tract infection in gamma interferon gene knockout mice. Infect Immun. 1997;65:2145–2152. doi: 10.1128/iai.65.6.2145-2152.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darville T, Andrews C W, Jr, Kishen L R, Rank R G, Williams D M. Transforming growth factor-α is associated with increased pathology in interferon-gamma gene knockout mice infected with chlamydiae. In: Stephens R S, Byrne G I, Christiansen G, Clarke I N, Grayston J T, Rank R G, Ridgway G L, Saikku P, Schachter J, Stamm W E, editors. Proceedings of the Ninth International Symposium on Human Chlamydial Infection. San Francisco, Calif: International Chlamydia Symposium; 1998. pp. 407–410. [Google Scholar]
- 11.Darville T, Andrews C W, Lafoon K K, Shymasani W, Kishen L R, Rank R G, Andrews C W, Jr, Laffoon K K. Mouse strain-dependent variation in the course and outcome of chlamydial genital tract infection is associated with differences in host response. Infect Immun. 1997;65:3065–3073. doi: 10.1128/iai.65.8.3065-3073.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Caterina R, Libby P, Peng H B, Thannickal V J, Rajavashisth T B, Gimbrone M A, Jr, Shin W S, Liao J K. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Investig. 1995;96:60–68. doi: 10.1172/JCI118074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Jong A L, Green D M, Trial J A, Birdsall H H. Focal effects of mononuclear leukocyte transendothelial migration: TNF- alpha production by migrating monocytes promotes subsequent migration of lymphocytes. J Leukoc Biol. 1996;60:129–136. doi: 10.1002/jlb.60.1.129. [DOI] [PubMed] [Google Scholar]
- 14.Fuller K A, Kanagawa O, Nahm M H. T cells within germinal centers are specific for the immunizing antigen. J Immunol. 1993;151:4505–4512. [PubMed] [Google Scholar]
- 15.Haraldsen G, Kvale D, Lien B, Farstad I N, Brandtzaeg P. Cytokine-regulated expression of E-selectin, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule- 1 (VCAM-1) in human intestinal microvascular endothelial cells. J Immunol. 1996;156:2558–2565. [PubMed] [Google Scholar]
- 16.Henninger D D, Panes J, Eppihimer M, Russell J, Gerritsen M, Anderson D C, Granger D N. Cytokine-induced VCAM-1 and ICAM-1 expression in different organs of the mouse. J Immunol. 1997;158:1825–1832. [PubMed] [Google Scholar]
- 17.Horie Y, Chervenak R P, Wolf R, Gerritsen M E, Anderson D C, Komatsu S, Granger D N. Lymphocytes mediate TNF-alpha-induced endothelial cell adhesion molecule expression: studies on SCID and RAG-1 mutant mice. J Immunol. 1997;159:5053–5062. [PubMed] [Google Scholar]
- 18.Igietseme J U, Rank R G. Susceptibility to reinfection after a primary chlamydial genital infection is associated with a decrease of antigen-specific T cells in the genital tract. Infect Immun. 1991;59:1346–1351. doi: 10.1128/iai.59.4.1346-1351.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ingalls R R, Rice P A, Qureshi N, Takayama K, Lin J S, Golenbock D T. The inflammatory cytokine response to Chlamydia trachomatis infection is endotoxin mediated. Infect Immun. 1995;63:3125–3130. doi: 10.1128/iai.63.8.3125-3130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johansson M, Schon K, Ward M, Lycke N. Genital tract infection with Chlamydia trachomatis fails to induce protective immunity in gamma interferon receptor-deficient mice despite a strong local immunoglobulin A response. Infect Immun. 1997;65:1032–1044. doi: 10.1128/iai.65.3.1032-1044.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karmann K, Min W, Fanslow W C, Pober J S. Activation and homologous desensitization of human endothelial cells by CD40 ligand, tumor necrosis factor, and interleukin 1. J Exp Med. 1996;184:173–182. doi: 10.1084/jem.184.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelly K A, Rank R G. Identification of homing receptors that mediate the recruitment of CD4 T cells to the genital tract following intravaginal infection with Chlamydia trachomatis. Infect Immun. 1997;65:5198–5208. doi: 10.1128/iai.65.12.5198-5208.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelly K A, Robinson E A, Rank R G. Initial route of antigen administration alters the T-cell cytokine profile produced in response to the mouse pneumonitis biovar of Chlamydia trachomatis following genital infection. Infect Immun. 1996;64:4976–4983. doi: 10.1128/iai.64.12.4976-4983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiviat N B, Wolner-Hanssen P, Peterson M, Wassenheit J, Stamm W E, Eschenbach D A, Paavonen J, Lingenfelter J, Bell T, Zabriskie V, Kirby B, Holmes K K. Localization of Chlamydia trachomatis infection by direct immunofluorescence and culture in pelvic inflammatory disease. Am J Obstet Gynecol. 1986;154:865–872. doi: 10.1016/0002-9378(86)90473-4. [DOI] [PubMed] [Google Scholar]
- 25.Lawson C, Ainsworth M, Yacoub M, Rose M. Ligation of ICAM-1 on endothelial cells leads to expression of VCAM-1 via a nuclear factor-kappa B-independent mechanism. J Immunol. 1999;162:2990–2996. [PubMed] [Google Scholar]
- 26.Marfaing-Koka A, Devergne O, Gorgone G, Portier A, Schall T J, Galanaud P, Emilie D. Regulation of the production of the RANTES chemokine by endothelial cells. Synergistic induction by IFN-gamma plus TNF-alpha and inhibition by IL-4 and IL-13. J Immunol. 1995;154:1870–1878. [PubMed] [Google Scholar]
- 27.Morrison R P, Feilzer K, Tumas D B. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect Immun. 1995;63:4661–4668. doi: 10.1128/iai.63.12.4661-4668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakabayashi T, Letterio J J, Geiser A G, Kong L, Ogawa N, Zhao W, Koike T, Fernandes G, Dang H, Talal N. Up-regulation of cytokine mRNA, adhesion molecule proteins, and MHC class II proteins in salivary glands of TGF-beta 1 knockout mice: MHC class II is a factor in the pathogenesis of TGF-beta 1 knockout mice. J Immunol. 1997;158:5527–5535. [PubMed] [Google Scholar]
- 29.Neumann B, Machleidt T, Lifka A, Pfeffer K, Vestweber D, Mak T W, Holzmann B, Kronke M. Crucial role of 55-kilodalton TNF receptor in TNF-induced adhesion molecule expression and leukocyte organ infiltration. J Immunol. 1996;156:1587–1593. [PubMed] [Google Scholar]
- 30.Pal S, Hui W, Peterson E M, de la Maza L M. Factors influencing the induction of infertility in a mouse model of Chlamydia trachomatis ascending genital tract infection. J Med Microbiol. 1998;47:599–605. doi: 10.1099/00222615-47-7-599. [DOI] [PubMed] [Google Scholar]
- 31.Parr M B, Parr E L. Langerhans cells and T lymphocyte subsets in the murine vagina and cervix. Biol Reprod. 1991;44:491–498. doi: 10.1095/biolreprod44.3.491. [DOI] [PubMed] [Google Scholar]
- 32.Parr M B, Parr E L. Mucosal immunity in the female and male reproductive tracts. In: Ogra P L, Lamm M E, McGhee J R, Mestecky J, Strober W, Bienenstock J, editors. Handbook of mucosal immunology. San Diego, Calif: Academic Press, Inc.; 1994. p. 677. [Google Scholar]
- 33.Patton D L, Kuo C-C, Wang S-P, Halbert S A. Distal tubal obstruction induced by repeated Chlamydia trachomatis salpingeal infection in pig-tailed macaques. J Infect Dis. 1987;155:1292–1299. doi: 10.1093/infdis/155.6.1292. [DOI] [PubMed] [Google Scholar]
- 34.Perry L L, Feilzer K, Caldwell H D. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-gamma-dependent and -independent pathways. J Immunol. 1997;158:3344–3352. [PubMed] [Google Scholar]
- 35.Perry L L, Feilzer K, Caldwell H D. Neither interleukin-6 nor inducible nitric oxide synthase is required for clearance of Chlamydia trachomatis from the murine genital tract epithelium. Infect Immun. 1998;66:1265–1269. doi: 10.1128/iai.66.3.1265-1269.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perry L L, Feilzer K, Portis J L, Caldwell H D. Distinct homing pathways direct T lymphocytes to the genital and intestinal mucosae in Chlamydia-infected mice. J Immunol. 1998;160:2905–2914. [PubMed] [Google Scholar]
- 37.Rank R G, Sanders M M, Kidd A T. Influence of the estrous cycle on the development of upper genital tract pathology as a result of chlamydial infection in the guinea pig model of pelvic inflammatory disease. Am J Pathol. 1993;142:1291–1296. [PMC free article] [PubMed] [Google Scholar]
- 38.Rasmussen S, Eckmann L, Quayle A J, Shen L, Zhang Y, Anderson D J, Fierer J, Stephens R S, Kagnoff M F. Secretion of proinflammatory cytokines by epithelial cells in response to Chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis. J Clin Investig. 1997;99:77–87. doi: 10.1172/JCI119136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-kappa B. Annu Rev Cell Biol. 1994;10:405–455. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- 40.Song S, Ling-Hu H, Roebuck K A, Rabbi M F, Donnelly R P, Finnegan A. Interleukin-10 inhibits interferon-gamma-induced intercellular adhesion molecule-1 gene transcription in human monocytes. Blood. 1997;89:4461–4469. [PubMed] [Google Scholar]
- 41.Stagg A J, Tuffrey M, Woods C, Wunderink E, Knight S C. Protection against ascending infection of the genital tract by Chlamydia trachomatis is associated with recruitment of major histocompatibility complex class II antigen-presenting cells into uterine tissue. Infect Immun. 1998;66:3535–3544. doi: 10.1128/iai.66.8.3535-3544.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stamm W E, Guinan M E, Johnson C, Starcher T, Holmes K K, McCormack W M. Effect of treatment regimens for Neisseria gonorrhoeae on simultaneous infection with Chlamydia trachomatis. N Engl J Med. 1984;310:545–549. doi: 10.1056/NEJM198403013100901. [DOI] [PubMed] [Google Scholar]
- 43.Streeter P R, Berg E L, Rouse B T N, Bargatze R F, Butcher E C. A tissue-specific endothelial cell molecule involved in lymphocyte homing. Nature. 1988;331:41–46. doi: 10.1038/331041a0. [DOI] [PubMed] [Google Scholar]
- 44.TerWee J, Sabara M, Kokjohn K, Sandbulte J, Frenchick P, Dreier K J. Characterization of the systemic disease and ocular signs induced by experimental infection with Chlamydia psittaci in cats. Vet Microbiol. 1998;59:259–281. doi: 10.1016/s0378-1135(97)00185-5. [DOI] [PubMed] [Google Scholar]
- 45.Tseng C K, Rank R G. Role of NK cells in the early host response to chlamydial genital infection. Infect Immun. 1998;66:5867–5875. doi: 10.1128/iai.66.12.5867-5875.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Westrom L, Joesoef R, Reynolds G, Hagdu A, Thompson S E. Pelvic inflammatory disease and fertility. A cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis. 1992;19:185–192. [PubMed] [Google Scholar]
- 47.Williams D M, Grubbs B G, Darville T, Kelly K, Rank R G. A role for interleukin-6 in host defense against murine Chlamydia trachomatis infection. Infect Immun. 1998;66:4564–4567. doi: 10.1128/iai.66.9.4564-4567.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wyrick P B, Knight S T, Paul T R, Rank R G, Barbier C S. Persistent chlamydial envelope antigens in antibiotic-exposed infected cells trigger neutrophil chemotaxis. J Infect Dis. 1999;179:954–966. doi: 10.1086/314676. [DOI] [PubMed] [Google Scholar]
- 49.Yamamoto M, Fujihashi K, Kawabata K, McGhee J R, Kiyono H. A mucosal intranet: intestinal epithelial cells down-regulate intraepithelial, but not peripheral, T lymphocytes. J Immunol. 1998;160:2188–2196. [PubMed] [Google Scholar]
- 50.Yang X, Gartner J, Zhu L, Wang S, Brunham R C. IL-10 gene knockout mice show enhanced Th1-like protective immunity and absent granuloma formation following Chlamydia trachomatis lung infection. J Immunol. 1999;162:1010–1017. [PubMed] [Google Scholar]