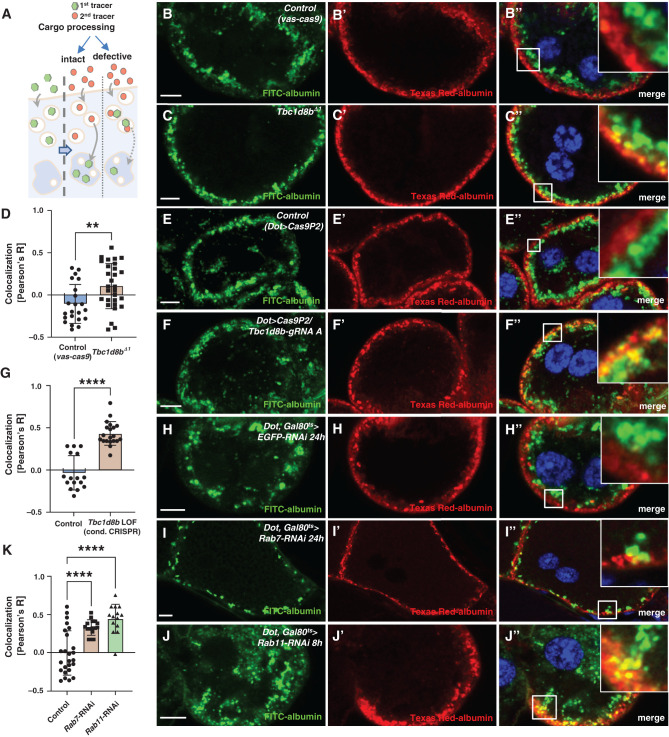
Figure 4.
Sequential tracer endocytosis indicates a role of Tbc1d8b for cargo processing. (A) Schematic illustrates the principle of the sequential tracer assay. The first tracer (green) is applied and endocytosed (left). Endocytic processing moves the first tracer to the deeper late endosomes during a chase period (middle). If endocytosis is defective both tracers will overlap in the endosomal sorting compartment (right). (B) and (C″) Sequential tracer endocytosis indicates a mild increase of colocalization between the first and second tracers for Tbc1d8bΔ1 nephrocytes (C)–(C″) compared with control cells (B)–(B″). Nuclei are marked by Hoechst 33342 in blue. (D) Quantitation of data analogous to (B) and (C) expressed as Pearson correlation coefficient for individual cells (mean±SD, n=8–12 animals per genotype, P<0.01) confirms increased colocalization of sequential tracers, suggesting delayed cargo processing. (E)–(F″) Sequential tracer endocytosis reveals enhanced colocalization of sequential tracers for CRISPR/Cas9-mediated loss-of-function of Tbc1d8b (F)–(F″) compared with control nephrocytes (E)–(E″). (G) Quantitation of data analogous to (E) and (F) expressed as Pearson correlation coefficient for individual cells (mean±SD, n=6–7 animals per genotype, P<0.0001) confirms increased tracer overlap indicating decelerated processing of endocytic cargo. (H)–(J″) Sequential tracer endocytosis in nephrocytes expressing Rab7-RNAi (I)–(I″) and Rab11-RNAi (J)–(J″) also show an elevated colocalization of first and second tracers compared with control cells (H)–(H″). Late endosomes appear smaller upon Rab7 silencing (I)–(I″) and are more diffuse on expression of Rab11-RNAi. (K) Quantitation of data analogous to (H)–(J) expressed as Pearson correlation coefficient for individual cells (mean±SD, n=8–12 animals per genotype, P<0.0001) supports enhanced colocalization by defective degradation and loss of Rab11.