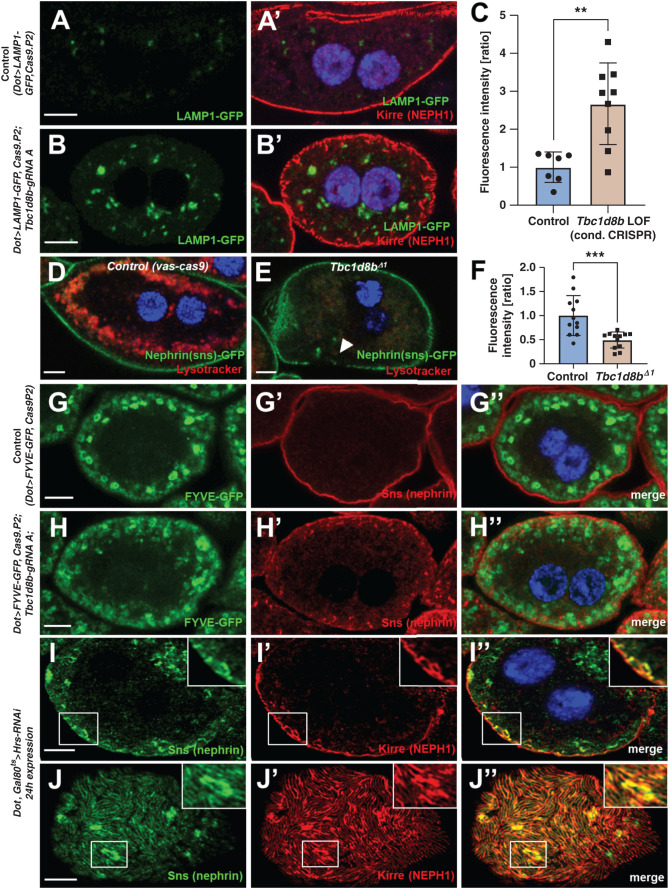
Figure 5.
Loss-of-function of Tbc1d8b results in delayed endosomal degradation. (A)–(B’) Nephrocytes expressing a GFP-LAMP1 fusion protein under control of UAS are costained for Kirre (NEPH1). The vesicles carrying the (unstained) reporter become brighter and expand on CRISPR/Cas9-mediated loss of Tbc1d8b (compare B to A). Nuclei are marked by Hoechst 33342 in blue. (C) Quantitation of data analogous to (A)–(B’) expressed as ratio of mean fluorescence intensity per animal (mean±SD, n=7–9 animals per genotype, P<0.01) supports accumulation of GFP-LAMP1 suggesting defective degradation. (D)–(E″) Shown are confocal images of living, unfixed nephrocytes exposed to lysotracker dye ex vivo. The cells express nephrin-GFP from a genome edited locus. Lysotracker-derived fluorescence intensity is diminished in Tbc1d8bΔ1 animals (E) compared with control (D). (F) Quantitation of lysotracker intensity in cells analogous to (D)–(E’) (gray value, mean±SD, n=12 animals per genotype, P<0.001) supports decreased acidification, in turn suggesting defective endosomal maturation. (G)–(H″) Nephrocytes expressing the reporter FYVE-GFP costained for nephrin show a more diffuse pattern of the vesicles positive for the (unstained) FYVE-GFP reporter comparing loss-of-function of Tbc1d8b (H)–(H″) with control cells (G)–(G″). The brightest and largest vesicles are missing, suggesting a reduced formation of PI3P on mature endosomes. (I)–(J″) Confocal microscopy images of a nephrocyte stained for slit diaphragm proteins are shown. A cell expressing Hrs-RNAi acutely for 24 hours shows accumulation of subcortical slit diaphragm protein in cross sections (I)–(I″), see also inset. Tangential sections reveal brighter sections within the lines of slit diaphragm protein that are blurry and partially confluent (J)–(J″).