Abstract
Background and objective
Different factors may influence colonoscopy performance measures. We aimed to analyze procedure‐ and endoscopist‐related factors associated with detection of colorectal lesions and whether these factors have a similar influence in the context of different colonoscopy indications: positive fecal immunochemical test (+FIT) and post‐polypectomy surveillance colonoscopies.
Methods
This multicenter cross‐sectional study included adults aged 40–80 years. Endoscopists (N = 96) who had performed ≥50 examinations were assessed for physician‐related factors. Adenoma detection rate (ADR), adenomas per colonoscopy rate (APCR), advanced ADR, serrated polyp detection (SDR), and serrated polyps per colonoscopy rate (SPPCR) were calculated.
Results
We included 12,932 procedures, with 4810 carried out after a positive FIT and 1967 for surveillance. Of the 96 endoscopists evaluated, 43.8% were women, and the mean age was 41.9 years. The ADR, advanced ADR, and SDR were 39.7%, 17.7%, and 12.8%, respectively. Adenoma detection rate was higher in colonoscopies after a +FIT (50.3%) with a more than doubled advanced ADR compared to non‐FIT procedures (27.6% vs. 13.0%) and similar results in serrated lesions (14.7% vs. 13.5%). Among all the detection indicators analyzed, withdrawal time was the only factor independently related to improvement (p < 0.001). Regarding FIT‐positive and surveillance procedures, for both indications, withdrawal time was also the only factor associated with a higher detection of adenomas and serrated polyps (p < 0.001). Endoscopist‐related factors (i.e., weekly hours dedicated to endoscopy, annual colonoscopy volume and lifetime number of colonoscopies performed) had also impact on lesion detection (APCR, advanced ADR and SPPCR).
Conclusions
Withdrawal time was the factor most commonly associated with improved detection of colonic lesions globally and in endoscopies for + FIT and post‐polypectomy surveillance. Physician‐related factors may help to address strategies to support training and service provision. Our results can be used for establishing future benchmarking and quality improvement in different colonoscopy indications.
Keywords: adenoma detection, colonoscopy, colorectal cancer, endoscopist, serrated polyp
Key summary.
Summarise the established knowledge on this subject
Different factors may influence colonoscopy performance measures, however, the relationship between these factors and colonoscopy quality is not always consistent, and whether the association would be consistent for different indications (i.e., surveillance colonoscopy) and different detection indicators is unknown.
What are the significant and/or new findings of this study?
Withdrawal time was the factor most commonly associated with improved detection of colonic lesions globally and in endoscopies for positive fecal immunochemical test (FIT) and post‐polypectomy surveillance.
Factors related to the endoscopist performing the colonoscopy had less influence than those related to the procedure. Additionally, a wide variation in detection rates among endoscopists was observed, even in colonoscopies because of +FIT. A national strategy supported by physician’ factors influencing lesion detection may help to reduce this large variability and improve quality of colonoscopies.
Minor differences were observed in factors influencing detection in colonoscopies after a positive FIT and for post‐polypectomy surveillance, and between adenomas and serrated polyps.
INTRODUCTION
Colonoscopy has become an essential tool in prevention and diagnosis of colorectal cancer (CRC) because it allows for detection and resection of premalignant lesions and even early CRC. However, its effectiveness can be limited by low‐quality procedures, leading to the identification of key performance measures over the years for achieving high‐quality colonoscopies. 1 , 2
Among quality indicators, the adenoma detection rate (ADR) is the most important because of its inverse association with the risk of interval cancer. 3 , 4 Factors that influence performance measures can be related to the procedure and to the physician performing the examination. Those factors directly associated with the endoscopic procedure have been widely addressed in primary screening colonoscopies and endoscopies after a positive fecal immunochemical test (FIT). Longer average times for withdrawal of the colonoscope and an adequate colon cleansing have been associated with higher adenoma detection rates. 5 , 6 , 7 Regarding endoscopist‐related factors, a wide variation in ADR has been documented. 8 , 9 , 10 For example, we previously reported a range for ADR of 4.5%–56.5% among 48 endoscopists performing primary screening colonoscopies. 11 This variation means that the development of metachronous adenomas depends not only on intrinsic biological factors but also on physician ability to detect adenomas. 12 Endoscopist specialty, the total number of lifetime colonoscopies performed, or personal factors have been also described as explaining this variation, especially for primary screening colonoscopies. 11 , 13 , 14 , 15
These reports have led to the development of an endoscopist profile focused on the ADR in different screening programs. 16 However, the relationship between these factors and colonoscopy quality is not always consistent, and whether the association would be consistent for different indications (i.e., surveillance colonoscopy) and different detection indicators is unknown. In this regard, serrated polyps are estimated to be the precursor of 15%–30% of CRC, but little is known about the influence of procedural‐ and physician‐related factors on detection of these polyps. 17 , 18
In this study, we analyzed the influence of factors related to the endoscopic procedure and physician characteristics in the detection of different colorectal lesions (adenomas and serrated polyps) across different indications for colonoscopy.
MATERIAL AND METHODS
Study characteristics and population
This multicenter and cross‐sectional study in 13 centers in Spain was nested within the QUALISCOPIA project. 19 Using consecutive sampling, we recruited adults aged 40–80 years, as either outpatients or hospital patients, who were undergoing a colonoscopy at these centers from February 2016 to December 2017. Exclusion criteria were a diagnosis of CRC or adenomas within the previous 6 months, having a colonoscopy to review an incomplete excision or piecemeal resection, emergency colonoscopies, endoscopic procedures to treat colon strictures or because of suspicion of abdominal or rectal mass, and having been diagnosed with inflammatory bowel disease or hereditary cancer syndrome. We also excluded procedures performed by residents.
According to the study aims, we considered FIT (OC‐Sensor, Eiken Chemical Co., Ltd; cut‐off level 20 μg/g) and post‐polypectomy surveillance to evaluate if endoscopic procedure and physician characteristics showed consistent associations in colonoscopies performed for specific indications.
This study was approved by the ethical review board of each participating center, and written informed consent was obtained from each included patient. Ethical board approval of this study was granted on 27 May 2015. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the institution's human research committee.
All information about patients' demographic data, procedures, pathology reports, or physicians was registered anonymously in the REDCap (Research Electronic Data Capture) database.
Characteristics of the procedures
The procedure‐related factors we identified were procedure indication, colonic cleansing, cecal intubation, withdrawal time, and sedation. Colon cleansing was described using the Boston Bowel Preparation Scale, and “adequate colon cleansing rate” was measured as the percentage of examinations that yielded two or three points in each colonic segment. “Cecal intubation rate” (CIR) was assessed as the percentage of colonoscopies reaching and visualizing the whole cecum and its landmarks. We reported adjusted CIR, excluding procedures with inadequate colon cleansing or impassable strictures. Colonoscopy withdrawal time was considered only in procedures without biopsy or therapy and calculated as the time from achievement of cecal intubation until the colonoscope was extracted through the anus. Moreover, data on the hospital, patient sex and age, preparation product, and time from the last dose of bowel preparation to the beginning of the colonoscopy were collected.
Endoscopist characteristics
For evaluation of physician quality, we included endoscopists performing ≥50 examinations in our study. Consistent with prior work, 11 a questionnaire about their personal characteristics and training was distributed to the participating endoscopists at the beginning of the study. Items recorded were age, sex, endoscopist specialty, years as a specialist, exclusive dedication to endoscopy practice, total number of colonoscopies performed during the career, number of colonoscopies performed during the previous year, hours per week dedicated to endoscopy, and participation in educational endoscopic activities during the previous 2 years. “Years as a specialist” was measured as the time from achieving the title of specialist to the start of the study. “Exclusive dedication to endoscopy practice” was defined as the physician's exclusively performing any type of endoscopy for at least 1 year prior to the start of the study. Each physician self‐reported total (lifelong) number of colonoscopies performed and colonoscopies performed during the previous year (annual colonoscopy volume) based on the starting date of the study. The number of hours per week dedicated to endoscopy was recorded for the year prior to the beginning of the study. Any meeting or course organized or supported by official scientific societies related to endoscopy that each physician participated in during the 2 years before the study began was considered an educational endoscopic activity. The endoscopists self‐reported all of these values.
Indicators of lesion detection
The categorical dependent variables were adenoma detection, advanced adenoma detection and serrated polyp detection (SDR), defined as the presence of an adenoma, an advanced adenoma or a serrated polyp, respectively. The quantitative dependent variables were adenoma per colonoscopy rate (APCR) described as the mean number of adenomas identified per colonoscopy and the serrated polyps per colonoscopy rate (SPPCR) defined as the mean number serrated polyps identified per colonoscopy. Adenoma detection rate, advanced ADR, or SDR were also assessed and described as the percentage of colonoscopies in which at least one conventional adenoma, advanced adenoma, or serrated polyp was detected in all included colonoscopies.
An adenoma was considered advanced when it was ≥10 mm, had tubulovillous or villous architecture, or had high‐grade dysplasia. For serrated polyps, we included sessile serrated lesions, traditional serrated adenomas of any size or location, and hyperplastic polyps ≥5 mm or proximal to rectosigmoid. Isolated rectosigmoid hyperplastic polyps <5 mm were not considered to be serrated polyps.
Statistical analysis
Categorical variables are reported as frequencies or percentages, continuous variables as means (standard deviations, SDs), and discrete variables as medians (25th–75th percentiles). Minimum and maximum observations are represented by ranges. Correlation was assessed using the bivariate Pearson correlation (r, correlation coefficient) for continuous variables and Spearman's correlation (r s, correlation coefficient) for discrete variables. In univariate analysis, binary logistic regression was used for categorical variables and for quantitative data. Variables with p < 0.1 were included in the multivariate analysis. The beta coefficient (β) expresses the degree of change in the outcome variable for every one unit of change in the predictor variable. Binary logistic regression analysis was used in the multivariate analysis for categorical variables and a Poisson mixed‐effects model was applied for quantitative data (APCR and SPPCR). Risk adjustment based on predictor variables (hospital, patient age and sex, and endoscopist) was performed. Odds ratios or β coefficients and their 95% confidence intervals were determined. Reported p values are two‐sided, and p < 0.05 was considered to indicate statistical significance. All calculations were performed using SPSS version 26.0 software (IBM Corp.).
RESULTS
Characteristics of eligible patients and procedures
A total of 12,932 procedures from 13 centers were included from February 2016 through December 2017. These procedures were performed in the same number of patients, and no repeat procedures were included. Slightly more than 50% of the patients were men (51.8%), and the largest fraction of individuals were aged 50–69 years old (Table 1). Regarding procedure indications, 5089 were performed because of gastrointestinal symptoms, 4810 after a +FIT, 1967 for post‐polypectomy surveillance, and 1066 as primary screening colonoscopies.
TABLE 1.
Characteristics of the 12,932 colonoscopies performed in the study
| Characteristics | Study procedures (N = 12,932 colonoscopies) |
|---|---|
| Hospital—no. (%) | |
| 1 | 1306 (10.1) |
| 2 | 1511 (11.7) |
| 3 | 1067 (8.3) |
| 4 | 188 (1.5) |
| 5 | 397 (3.1) |
| 6 | 1083 (8.4) |
| 7 | 1882 (14.6) |
| 8 | 119 (0.9) |
| 9 | 1687 (13.0) |
| 10 | 969 (7.5) |
| 11 | 653 (5.0) |
| 12 | 847 (6.5) |
| 13 | 1223 (9.5) |
| Sex—no. (%) | |
| Male | 6703 (51.8) |
| Female | 6229 (48.2) |
| Age—no. (%) | |
| 40–49 years | 1426 (11.0) |
| 50–59 years | 3726 (28.8) |
| 60–69 years | 5097 (39.4) |
| 70–80 years | 2683 (20.8) |
| Indication—no. (%) | |
| Gastrointestinal symptoms | 5089 (39.4) |
| +FIT | 4810 (37.2) |
| Post‐polypectomy surveillance | 1967 (15.2) |
| Primary screening colonoscopy | 1066 (8.2) |
| Preparation product—no. (%) | |
| PEG 4L | 3155 (24.4) |
| PEG 2L + ascorbate | 4656 (36.0) |
| SPMC | 5043 (39.0) |
| Other products | 78 (0.6) |
| Time between preparation and colonoscopy—median (25th–75th percentiles) | 4 (4–6) |
| Adequate colon cleansing rate—no. (%) | 11,199 (86.6) |
| Cecal intubation rate—no. (%) | 12,334 (95.4) |
| Withdrawal time—median (25th–75th percentiles), minutes | 8 (6–10) |
| Sedation rate—no. (%) | 11,983 (92.7) |
| Professional administering sedation—no. (%) | |
| Endoscopist | 9814 (81.9) |
| Anesthesiologist | 1605 (13.4) |
| Other | 564 (4.7) |
| Sedation regimen—no. (%) | |
| Propofol sedation | 8783 (73.3) |
| Conscious sedation | 3200 (26.7) |
Abbreviations: +FIT, positive fecal immunochemical test; PEG, polyethylene glycol; SPMC, sodium picosulfate with magnesium citrate.
Endoscopist characteristics and quality indicators
Of the 96 physicians included in the study, 42 (43.8%) were women, and the mean age was 41.9 years (SD ± 9.8) (Table 2). All were gastroenterologists from tertiary hospitals, with an average of 12.8 years (SD ± 9.8) as a specialist. Thirty endoscopists (32.3%) reported exclusive dedication to endoscopy practice. The lifetime number of colonoscopies performed ranged from 800 to 40,000, and 63.5% of physicians performed ≥500 colonoscopies in a year. The mean hours per week dedicated to endoscopy was 23.8 (SD ± 12.4).
TABLE 2.
Characteristics of the 96 endoscopists participating in the study
| Characteristics | Study population (N = 96) |
|---|---|
| Sex—no. (%) | |
| Male | 54 (56.3) |
| Female | 42 (43.8) |
| Age | |
| Mean ± SD, years | 41.9 ± 9.8 |
| Range | 27–65 |
| Gastroenterologist—no. (%) | 96 (100) |
| Tertiary hospital—no. (%) | 96 (100) |
| Years as specialist | |
| Mean ± SD | 12.8 ± 9.8 |
| Range | 1–36 |
| Exclusive dedication to endoscopy practice—no. (%) | |
| Yes | 30 (32.3) |
| No | 63 (67.7) |
| Life‐long number of colonoscopies performed | |
| Median (25th–75th percentiles) | 5000 (2400–12000) |
| Range | 800–40000 |
| Annual colonoscopy volume—no. (%) | |
| <500 colonoscopies | 35 (36.5) |
| ≥500 colonoscopies | 61 (63.5) |
| Weekly hours dedicated to endoscopy | |
| Mean ± SD | 23.8 ± 12.4 |
| Range | 2–65 |
| Educational endoscopic activities in previous 2 years—no. (%) | 85 (88.5) |
Abbreviation: SD, standard deviation.
Regarding quality indicators, the mean ADR, advanced ADR, and SDR of the 96 endoscopists participating in the study were 39.7%, 17.7%, and 12.8%, respectively (Table 3). As expected, the APCR was higher than the SPPCR (0.97 vs. 0.22). Additionally, we observed a high degree of correlation between the ADR and the APCR (r s = 0.852, p < 0.001) and the SDR and SPPCR (r s = 0.870, p < 0.001) (Supplementary Figure 1). Quality indicators of +FIT and non‐FIT procedures are also reported in Table 3. The highest ADR was reached in colonoscopies after a +FIT (50.3%) with a more than doubled advanced ADR compared to non‐FIT procedures (+FIT: 27.6%; non‐FIT: 13.0%). Otherwise, similar results were obtained among serrated lesions (+FIT SDR: 14.7%; non‐FIT SDR: 13.5%). Distribution of findings according to the indications included are shown in Figure 1 (Supplementary Table 1).
TABLE 3.
Quality indicators of the 96 endoscopists participating in the study including all procedures, colonoscopies after a +FIT and non‐FIT procedures
| Global | FIT | Non‐FIT procedures | ||||
|---|---|---|---|---|---|---|
| Quality indicator | Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | Range |
| ADR—% | 39.7 (12.0) | 13.2–74.0 | 50.3 (18.9) | 0.0–100.0 | 35.8 (13.3) | 13.8–100.0 |
| APCR | 0.97 (0.45) | 0.18–2.42 | 1.31 (0.69) | 0.0–3.51 | 0.80 (0.38) | 0.21–2.0 |
| Advanced ADR—% | 17.7 (10.9) | 2.4–61.0 | 27.6 (18.1) | 0.0–100.0 | 13.0 (11.8) | 0.0–100.0 |
| SDR—% | 12.8 (5.8) | 3.3–27.4 | 14.7 (10.6) | 0.0–43.5 | 13.5 (12.0) | 0.0–100.0 |
| SPPCR | 0.22 (0.12) | 0.05–0.72 | 0.23 (0.19) | 0.0–0.96 | 0.22 (0.16) | 0.0–0.82 |
Abbreviations: ADR, adenoma detection rate; APCR, adenoma per colonoscopy rate; FIT, fecal immunochemical test; SD, standard deviation; SDR, serrated polyp detection rate; SPPCR, serrated polyps per colonoscopy rate.
FIGURE 1.
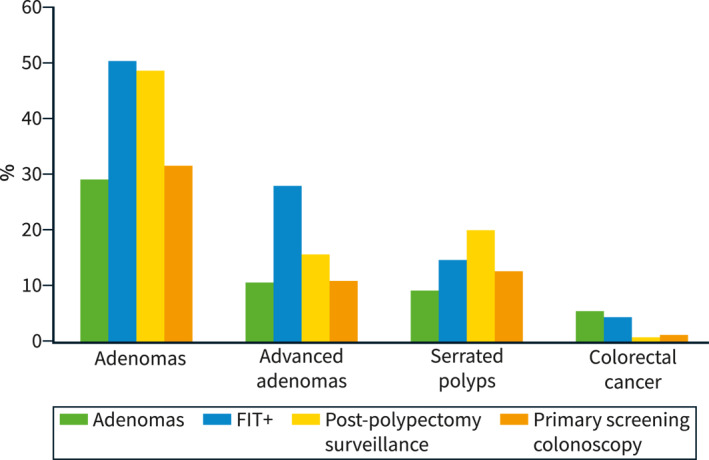
Detection rate of lesions according to indication
Factors related to lesion detection
Considering the whole cohort of 12,932 procedures, results of the univariate analysis can be seen in Supplementary Table 2. In the multivariate analysis, withdrawal time was associated with an increase in all indicators of lesion detection: adenoma detection (OR: 1.39; 95%CI 1.34–1.43); advanced adenoma detection (OR: 1.36; 95%CI 1.30–1.41), SDR (OR: 1.17; 95%CI 1.13–1.22), APCR (β: 0.146; 95%CI 0.128–0.164), and SPPCR (β: 0.181; 95%CI 0.145–0.216) (Table 4). Regarding adenomas, an adequate colon cleansing and reaching the cecum also were factors in improved detection, but for serrated polyps, only physician age was inversely associated with serrated polyps detection. Adenoma, advanced adenoma and SDR according to different withdrawal time intervals can be seen in Figure 2.
TABLE 4.
Multivariate analysis of procedure‐ and endoscopist‐related factors and their association with quality indicators of lesion detection
| Adenoma detection | ||
|---|---|---|
| OR (95%CI) | p | |
| Adequate colon cleansing (yes; no) | 1.16 (1.35–1.47) | 0.025 |
| Cecal intubation (yes; no) | 2.82 (1.54–5.17) | 0.001 |
| Withdrawal time | 1.39 (1.34–1.43) | <0.001 |
| APCR | ||
|---|---|---|
| β coefficient (95%CI) | p | |
| Adequate colon cleansing (yes) | 0.179 (0.113–0.245) | <0.001 |
| Cecal intubation (yes) | 0.701 (0.347–1.054) | <0.001 |
| Withdrawal time | 0.146 (0.128–0.164) | <0.001 |
| Annual colonoscopy volume (≥500) | 0.076 (0.012–0.139) | 0.020 |
| Weekly hours dedicated to endoscopy | 0.004 (0.001–0.006) | 0.008 |
| Advanced adenoma detection | ||
|---|---|---|
| OR (95%CI) | p | |
| Adequate colon cleansing (yes; no) | 1.19 (1.01–1.41) | 0.038 |
| Withdrawal time | 1.36 (1.30–1.41) | <0.001 |
| Endoscopist sex (male; female) | 1.26 (1.11–1.43) | <0.001 |
| Endoscopist age | 0.98 (0.97–0.99) | <0.001 |
| Lifetime number of colonoscopies performed | 1.00 (1.00–1.00) | <0.001 |
| Serrated polyp detection | ||
|---|---|---|
| OR (95%CI) | p | |
| Withdrawal time | 1.17 (1.13–1.22) | <0.001 |
| Endoscopist age | 0.99 (0.98–0.99) | 0.010 |
| SPPCR | ||
|---|---|---|
| β coefficient (95%CI) | p | |
| Withdrawal time | 0.181 (0.145–0.216) | <0.001 |
| Endoscopist age | −0.013 (−0.023–0.004) | 0.005 |
| Lifetime number of colonoscopies performed | −1.92 × 10−5 (−3.02 × 10−5–8.26 × 10−6) | 0.001 |
| Weekly hours dedicated to endoscopy | 0.014 (0.010–0.018) | <0.001 |
Abbreviations: APCR, adenoma per colonoscopy rate; CI, confidence interval; OR, odds ratio; SPPCR, serrated polyps per colonoscopy rate.
FIGURE 2.
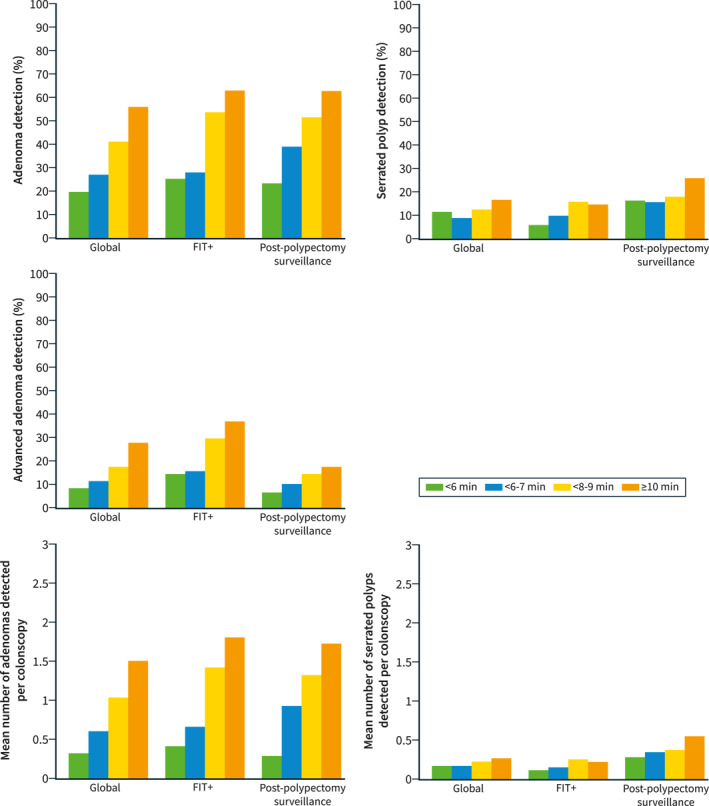
Adenoma, advanced adenoma and serrated polyp detection according to different withdrawal time intervals (<6 min, 6–7 min, 8–9 min and ≥10 min) in all procedures, in colonoscopies after + fecal immunochemical test (FIT) and in procedures because of post‐polypectomy surveillance
Factors related to lesion detection in +FIT based procedures
Results of the univariate analysis can be seen in Supplementary Table 3. After adjustment by hospital, patient age and sex, and endoscopist, only withdrawal time was associated (p < 0.001) with a higher detection rate of adenomas and serrated polyps, affecting all of the analyzed indicators: adenoma detection (OR: 1.44; 95%CI 1.37–1.50), APCR (β: 0.133; 95%CI 0.106–0.160), advanced adenoma detection (OR: 1.32; 95%CI 1.26–1.39), SDR (OR: 1.11; 95%CI 1.06–1.16), and SPPCR (β: 0.172; 95%CI 0.125–0.200; Table 5). Additionally, CIR was associated with an increase in detection rates for all but advanced adenomas (p < 0.05). Weekly hours dedicated to endoscopy showed a small association with increased adenoma detection (OR: 1.02; 95%CI 1.02–1.03), APCR (β: 0.006; 95%CI 0.001–0.010), and advanced adenoma detection (OR: 1.02; 95%CI 1.02–1.03).
TABLE 5.
Multivariate analysis of procedure‐ and endoscopist‐related characteristics associated with quality indicators of lesion detection in FIT‐based procedures and post‐polypectomy surveillance
| Adenoma detection | ||||
|---|---|---|---|---|
| FIT‐based colonoscopies | Post‐polypectomy surveillance | |||
| OR (95%CI) | p | OR (95%CI) | p | |
| Cecal intubation (yes; no) | 7.55 (1.63–34.99) | 0.010 | – | – |
| Withdrawal time | 1.44 (1.37–1.50) | <0.001 | 1.29 (1.19–1.39) | <0.001 |
| Endoscopist age | 1.02 (1.01–1.04) | 0.001 | – | – |
| Weekly hours dedicated to endoscopy | 1.02 (1.01–1.03) | 0.001 | – | – |
| APCR | ||||
|---|---|---|---|---|
| FIT‐based colonoscopies | Post‐polypectomy surveillance | |||
| β coefficient (95%CI) | p | β coefficient (95%CI) | p | |
| Adequate colon cleansing (yes) | 0.133 (0.019–0.246) | 0.022 | – | – |
| Cecal intubation (yes) | 0.771 (0.115–1.426) | 0.021 | – | – |
| Withdrawal time | 0.133 (0.106–0.160) | <0.001 | 0.164 (0.119–0.210) | <0.001 |
| Weekly hours dedicated to endoscopy | 0.006 (0.001–0.010) | 0.011 | – | – |
| Annual colonoscopy volume (≥500) | – | – | 0.319 (0.170–0.468) | <0.001 |
| Advanced adenoma detection | ||||
|---|---|---|---|---|
| FIT‐based colonoscopies | Post‐polypectomy surveillance | |||
| OR (95%CI) | p | OR (95%CI) | p | |
| Withdrawal time | 1.32 (1.26–1.39) | <0.001 | 1.16 (1.04–1.29) | 0.009 |
| Weekly hours dedicated to endoscopy | 1.02 (1.01–1.03) | 0.001 | – | – |
| Serrated polyp detection | ||||
|---|---|---|---|---|
| FIT‐based colonoscopies | Post‐polypectomy surveillance | |||
| OR (95%CI) | p | OR (95%CI) | p | |
| Cecal intubation (yes; no) | 3.44 (1.07–11.10) | 0.039 | – | – |
| Withdrawal time | 1.11 (1.06–1.16) | <0.001 | 1.15 (1.04–1.27) | 0.006 |
| Endoscopist age | – | – | 0.97 (0.95–0.99) | 0.004 |
| SPPCR | ||||
|---|---|---|---|---|
| FIT‐based colonoscopies | Post‐polypectomy surveillance | |||
| β coefficient (95%CI) | p | β coefficient (95%CI) | p | |
| Adequate colon cleansing (yes) | – | – | 0.333 (0.079–0.586) | 0.010 |
| Cecal intubation (yes; no) | 1.450 (0.312–2.586) | 0.012 | – | – |
| Withdrawal time | 0.172 (0.125–0.220) | <0.001 | 0.241 (0.163–0.319) | <0.001 |
| Endoscopist age | – | – | −0.019 (−0.035 to −0.003) | 0.018 |
Abbreviations: APCR, adenoma per colonoscopy rate; CI, confidence interval; OR, odds ratio; SPPCR, serrated polyps per colonoscopy rate.
Factors related to lesion detection in post‐polypectomy surveillance procedures
Regarding quality metrics of endoscopists among surveillance procedures, the ADR was 48.7% (SD, 21.6) and APCR was 1.15 (SD, 0.69) similar to FIT + procedures (Table 3). On the contrary, the advanced adenoma detection rate was 15.9%, almost half of that found among FIT colonoscopies which means that unlike in FIT + patients, the majority of adenomas found were non‐advanced. Regarding serrated lesions, SDR was 20.3% (SD, 20.2) and SPPCR was 0.45 (Supplementary Table 1).
Results of the univariate analysis can be seen in Supplementary Table 4. In the multivariate analysis, withdrawal time was linked to increases in all of the analyzed indicators of detection, either for adenomas or serrated polyps: adenoma detection (OR: 1.29; 95%CI 1.19–1.39), APCR (β: 0.164; 95%CI 0.119–0.210), advanced adenoma detection (OR: 1.16; 95%CI 1.04–1.29), SDR (OR: 1.15; 95%CI 1.04–1.27), and SPPCR (β: 0.241; 95%CI 0.163–0.319). As with the results for the other analyses, younger endoscopists were more likely to detect serrated lesions. Additionally, adequate colon cleansing was related to SPPCR (β: 0.333; 95%CI 0.079–0.586) and annual colonoscopy volume to APCR (β: 0.319; 95%CI 0.170–0.468) (Table 5).
DISCUSSION
The main finding of this study is that withdrawal time was the factor most commonly associated with improved detection of colonic lesions, among procedures overall and for the two specific indications evaluated. When analyzed together with other modifiable factors or endoscopist‐related factors, withdrawal time was consistently associated with all adenoma and SDR as well as with adenoma and SPPCR rates. This influence of withdrawal time also was consistently associated with all of the indicators of lesion detection in analyses of procedures for the two selected indications. Additionally, factors related to the endoscopist performing the colonoscopy had less influence than those related to the procedure, otherwise, there is still chance to address strategies to support training and service provision. Finally, we found some minor differences in factors influencing detection in colonoscopies after a positive FIT and for post‐polypectomy surveillance, and between adenomas and serrated polyps, which could suggest a need to focus on each of them separately.
Evidence from the literature solidly supports the role of withdrawal time as a main factor in lesion detection, yet this quality indicator has been controversial. Here we sought to comprehensively analyze different factors associated with adenoma and SDR to clearly delineate truly relevant versus secondary influences. We also evaluated modifiable procedure‐related factors as well as factors related to the characteristics and practice of the endoscopists. In previous similar studies, results have been controversial. Adler et al found no association between withdrawal time and adenoma detection in a large cohort of primary screening colonoscopies in Germany (12,134 procedures). 20 Some other studies also found no increase in polyp detection improvement with recording withdrawal time, and additional studies found no benefit of using a threshold time to predict the likelihood of finding a polyp. 21 , 22 , 23 More recently, however, withdrawal time has been associated with ADR and inversely associated with interval CRC and also related to detection of lesions such as serrated polyps. 5 , 7 , 24 , 25 , 26 Based on these findings, two of the major international endoscopy societies considered including this indicator as a key performance measure to improve colonoscopy quality. 1 , 2
The vast majority of studies underscoring the role of withdrawal time have been performed in primary screening colonoscopies. In 2019, Cavichi et al evaluated patient‐ and physician‐dependent factors in ADR and SDR. In their study, they included indications in addition to the screening procedures, such as personal or familial history of CRC (43.5%), digestive symptoms (37.3%), or + FIT (6.2%). In their multivariate analysis, they found that withdrawal time was related only to SDR, but they did not differentiate by indication. 27 In our study, withdrawal time was the only factor associated with lesion detection either globally or by specific indication, which supports previously published results and adds to them with data related to specific indications, that is, FIT‐based procedures and post‐polypectomy surveillance. The median withdrawal time for both indications in our study was 8 min in normal colonoscopies. This value could be an overestimate, especially for surveillance procedures, because physicians could be aware of a quality audit; however, similar results already have been reported. 5 , 7 , 24 , 25 , 26 Although our data do not support setting a threshold of time, special attention to withdrawing the colonoscope from the cecum must be applied for all indications. This attention is warranted by the close association among withdrawal time, increased lesion detection, and colonoscopy quality and could reduce metachronous lesions.
In general, our findings show that apart from withdrawal time, other modifiable colonoscopy factors such as colonic cleansing and cecal intubation were the most consistently related to both adenoma and SDR. On the other hand, endoscopist factors seemed to be less relevant, with only a modest association of weekly hours performing endoscopy, an annual volume >500 colonoscopies, and lifetime number of colonoscopies performed. Age was also associated with better detection, especially of serrated polyps, with higher rates in “younger” physicians. These results are in agreement with those already reported and support the fact that “young” endoscopists are more familiar with and aware of serrated polyps. 28 Although detection rates, especially in FIT screening, exceed the minimum requirements recommended by clinical practice guidelines, 2 we have not pre‐selected our endoscopists. The only requirement was to have completed residency training. Therefore, the endoscopists included represent a wide range of the current scenario in Spain. On the one hand, this could explain the wide ranges observed in detection rates, and on the other, could justify the small effect of the endoscopist‐related factors in detecting lesions. Thus, these findings might be different when analyzed in a more homogeneous cohort of physicians. Additionally, our results highlight the need to address strategies to support training, even in “older” physicians, to detect and recognize lesions, as well as service provision in order to reduce this great variety in physician's detection ability.
Since the development of population‐based CRC screening programs, key performance measures for colonoscopy have been developed to achieve high‐quality procedures. 1 , 2 The increased importance of this concept has led to the development of a specific profile with minimum requirements for performing this type of procedure, for example, in the Spanish CRC screening program. 16 However, most of the fulfillment and benchmarking information for quality indicators has been obtained from primary screening colonoscopies, and secondarily extended to + FIT procedures. 1 , 2 , 29 Previous research from our group evaluated colonoscopy performance measures according to procedure indication. As expected, we observed substantial variation by indication, and our results highlighted the need to address performance measures that could be extended to other colonoscopy indications, going beyond primary screening procedures. 19 In the present study, we have assessed two indications separately and examined factors that may influence detection indicators. We found some small differences in factors related to detection with respect to the indication. However, the variation in these factors was not especially substantial, particularly taking into account the important differences between detection rates in both indications, with more advanced lesions in +FIT colonoscopies and mostly small and irrelevant lesions in surveillance colonoscopies. 19 Here, it is of note that the key role of withdrawal time was consistent across all detection rates evaluated for both indications.
We also found some small differences between factors related to the detection of adenomas and serrated polyps. In general, adenoma detections were more related to modifiable factors, whereas the detection for serrated polyps were more related to endoscopist factors. Our results show a high correlation between adenoma or SDR and the rates of adenomas or serrated polyps per colonoscopy, demonstrating that these measures are different sides of the same coin and reflect the same skills of good detectors.
Regarding detection rates according to patient age groups, the results obtained in the 40–49 age group are notable, especially in FIT + procedures, where the ADR is 30.8% and the rate of advanced adenomas is 6.5%. In Spain, population‐based CRC screening is aimed to individuals between 50 and 69 years old. In our study, individuals <50 years with +FIT were out of screening programs, therefore, FIT in this population was requested following physician criteria (e.g., symptoms, family history of CRC of adenomatous polyps…) Despite we didn't identify the reason to request fecal test and we cannot stablish solid conclusions based on our data, we must remain vigilant as there is an increasing tendency to lower the starting age for CRC screening. 30
Some limitations of our study need to be addressed. Because of its design, a cause‐and‐effect relationship cannot be established. Participant endoscopists calculated their number of colonoscopies performed, which could be a limitation; however, these self‐calculated numbers correlated with the number of procedures performed in the study. Withdrawal time was also self‐recorded, and inequities might exist. The participating centers are academic centers, which can lead to overestimation of quality indicators. Data on insufflation techniques have not been included in our study, so the influence of water, air or carbon dioxide on lesion detection cannot be addressed, and may result in inaccuracies of detection rates. Moreover, we did not include some patient factors such as comorbidities, body mass index, or smoking history because we considered the age and sex case‐mix to be the most relevant factors in detection of colonic lesions. Additionally, surveillance procedures included in our study have followed recommendations from clinical practice guidelines prior to 2020. 31 However, new recommendations published lately 32 may impact quality metrics, so this aspect should be explored in the future.
In conclusion, we identified withdrawal time as the only factor consistently influencing detection of adenomas and serrated polyps in general colonoscopies and colonoscopies for the specific indications of +FIT and post‐polypectomy surveillance. Other modifiable factors, such as cecal intubation and colonic cleansing, had some modest influence. Physician‐related factors may help to address strategies to support training and service provision. Our study provides real‐world data on lesion detection metrics in colonoscopy which could be used for future benchmarking and quality improvement.
AUTHOR CONTRIBUTIONS
Carolina Mangas‐Sanjuan and Rodrigo Jover were involved with the study concept and design and drafting of the manuscript; Carolina Mangas‐Sanjuan, Agustin Seoane, Marco Antonio Alvarez‐Gonzalez, Alberto Luè, Adolfo Suárez, Verónica Álvarez‐García, Luis Bujanda, sabel Portillo, Natalia González, Lucía Cid‐Gomez, Joaquín Cubiella, Elena Rodríguez‐Camacho, Marta Ponce, Pilar Díez‐Redondo, Maite Herráiz, María Pellisé, and Akiko Ono were involved with acquisition of data; Carolina Mangas‐Sanjuan, Pedro Zapater, and Rodrigo Jover were involved with analysis, interpretation of data, and statistical analysis. All authors were involved with critical revision of the manuscript for important intellectual content and final approval of the article.
CONFLICT OF INTEREST
The author declares that they have no conflict of interest.
Supporting information
Supporting Information S1
Supporting Information S2
Supporting Information S3
ACKNOWLEDGMENTS
This work was supported by the Instituto de Salud Carlos III (PI14/01386, PI17/01756), Instituto de Investigación Sanitaria y Biomédica de Alicante Foundation ISABIAL (UGP‐14‐120, UGP‐14‐265, UGP‐17), Asociación Española de Gastroenterología (AEG‐Beca grupos de trabajo 2016), and the Asociación Española Contra el Cáncer (Fundación Científica GCB13131592CAST). Asociación para la Investigación en Gastroenterología de la Provincia de Alicante (AIGPA), a private association that promotes research in gastrointestinal diseases in Alicante, also supported the logistical aspects of the study but declares no conflict of interest.
Mangas‐Sanjuan C, Seoane A, Alvarez‐Gonzalez MA, Luè A, Suárez A, Álvarez‐García V, et al. Factors associated with lesion detection in colonoscopy among different indications. United European Gastroenterol J. 2022;10(9):1008–19. 10.1002/ueg2.12325
DATA AVAILABILITY STATEMENT
Research data are not shared.
REFERENCES
- 1. Rex DK, Schoenfeld PS, Cohen J, Pike IM, Adler DG, Fennerty MB, et al. Quality indicators for colonoscopy. Gastrointest Endosc. 2015;81(1):31–53. 10.1016/j.gie.2014.07.058 [DOI] [PubMed] [Google Scholar]
- 2. Kaminski MF, Thomas‐Gibson S, Bugajski M, Bretthauer M, Rees C, Dekker E, et al. Performance measures for lower gastrointestinal endoscopy: a European Society of Gastrointestinal Endoscopy (ESGE) quality improvement initiative. Endoscopy. 2017;49(4):378–97. 10.1055/s-0043-103411 [DOI] [PubMed] [Google Scholar]
- 3. Kaminski MF, Regula J, Kraszewska E, Polkowski M, Wojciechowska U, Didkowska J, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362(19):1795–803. 10.1056/nejmoa0907667 [DOI] [PubMed] [Google Scholar]
- 4. Corley DA, Jensen CD, Marks AR, Zhao WK, Lee JK, Doubeni CA, et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370(14):1298–306. 10.1056/nejmoa1309086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barclay RL, Vicari JJ, Doughty AS, Johanson JF, Greenlaw RL. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med. 2006;355(24):2533–41. 10.1056/nejmoa055498 [DOI] [PubMed] [Google Scholar]
- 6. Froehlich F, Wietlisbach V, Gonvers JJ, Burnand B, Vader JP. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc. 2005;61(3):378–84. 10.1016/s0016-5107(04)02776-2 [DOI] [PubMed] [Google Scholar]
- 7. Jover R, Zapater P, Polania E, Bujanda L, Lanas A, Hermo JA, et al. Modifiable endoscopic factors that influence the adenoma detection rate in colorectal cancer screening colonoscopies. Gastrointest Endosc. 2013;77(3):381–9. 10.1016/j.gie.2012.09.027 [DOI] [PubMed] [Google Scholar]
- 8. Shaukat A, Oancea C, Bond JH, Church TR, Allen JI. Variation in detection of adenomas and polyps by colonoscopy and change over time with a performance improvement program. Clin Gastroenterol Hepatol. 2009;7(12):1335–40. 10.1016/j.cgh.2009.07.027 [DOI] [PubMed] [Google Scholar]
- 9. Imperiale TF, Glowinski EA, Juliar BE, Azzouz F, Ransohoff DF. Variation in polyp detection rates at screening colonoscopy. Gastrointest Endosc. 2009;69(7):1288–95. 10.1016/j.gie.2007.11.043 [DOI] [PubMed] [Google Scholar]
- 10. Boroff ES, Gurudu SR, Hentz JG, Leighton JA, Ramirez FC. Polyp and adenoma detection rates in the proximal and distal colon. Am J Gastroenterol. 2013;108(6):993–9. 10.1038/ajg.2013.68 [DOI] [PubMed] [Google Scholar]
- 11. Jover R, Zapater P, Bujanda L, Hernandez V, Cubiella J, Pellise M, et al. Endoscopist characteristics that influence the quality of colonoscopy. Endoscopy. 2016;48(3):241–7. 10.1055/s-0042-100185 [DOI] [PubMed] [Google Scholar]
- 12. Mangas‐Sanjuan C, Zapater P, Cubiella J, Murcia O, Bujanda L, Hernandez V, et al. Importance of endoscopist quality metrics for findings at surveillance colonoscopy: the detection‐surveillance paradox. United Eur Gastroenterol J. 2018;6(4):622–9. 10.1177/2050640617745458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rabeneck L, Paszat LF, Saskin R. Endoscopist specialty is associated with incident colorectal cancer after a negative colonoscopy. Clin Gastroenterol Hepatol. 2010;8(3):275–9. 10.1016/j.cgh.2009.10.022 [DOI] [PubMed] [Google Scholar]
- 14. Mehrotra A, Morris M, Gourevitch RA, Carrell DS, Leffler DA, Rose S, et al. Physician characteristics associated with higher adenoma detection rate. Gastrointest Endosc. 2018;87(3):778–86. 10.1016/j.gie.2017.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ezaz G, Leffler DA, Beach S, Schoen RE, Crockett SD, Gourevitch RA, et al. Association between endoscopist personality and rate of adenoma detection. Clin Gastroenterol Hepatol. 2019;17(8):1571–9. 10.1016/j.cgh.2018.10.019 [DOI] [PubMed] [Google Scholar]
- 16. Grupo de trabajo AEG‐SEED. Programa de calidad en colonoscopia de cribado. Guía de práctica clínica de Calidad en la colonoscopia de cribado del cáncer colorrectal. Madrid: EDIMSA. Editores Médicos, S.A.; 2011. [Google Scholar]
- 17. Toyota M, Ahuja N, Ohe‐Toyota M, Herman JG, Baylin SB, Issa JPJ. CPG island methylator phenotype in colorectal cancer. Med Sci. 1999;96(15):8681–6. 10.1073/pnas.96.15.8681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 2007;50(1):113–30. 10.1111/j.1365-2559.2006.02549.x [DOI] [PubMed] [Google Scholar]
- 19. Mangas‐Sanjuan C, Santana E, Cubiella J, Rodriguez‐Camacho E, Seoane A, Alvarez‐Gonzalez MA, et al. Variation in colonoscopy performance measures according to procedure indication. Clin Gastroenterol Hepatol. 2020;18(5):1216–23. 10.1016/j.cgh.2019.08.035 [DOI] [PubMed] [Google Scholar]
- 20. Adler A, Wegscheider K, Lieberman D, Aminalai A, Aschenbeck J, Drossel R, et al. Factors determining the quality of screening colonoscopy: a prospective study on adenoma detection rates, from 12, 134 examinations [Berlin colonoscopy project 3, BECOP‐3]. Gut. 2013;62(2):236–41. 10.1136/gutjnl-2011-300167 [DOI] [PubMed] [Google Scholar]
- 21. Moritz V, Bretthauer M, Ruud HK, Glomsaker T, de Lange T, Sandvei P, et al. Withdrawal time as a quality indicator for colonoscopy: a nationwide analysis. Endoscopy. 2012;44(05):476–81. 10.1055/s-0032-1306898 [DOI] [PubMed] [Google Scholar]
- 22. Taber A, Romagnuolo J. Effect of simply recording colonoscopy withdrawal time on polyp and adenoma detection rates. Gastrointest Endosc. 2010;71(4):782–6. 10.1016/j.gie.2009.12.008 [DOI] [PubMed] [Google Scholar]
- 23. Sawhney MS, Cury MS, Neeman N, Ngo LH, Lewis JM, Chuttani R, et al. Effect of institution‐wide policy of colonoscopy withdrawal time≥7 minutes on polyp detection. Gastroenterology. 2008;135(6):1892–8. 10.1053/j.gastro.2008.08.024 [DOI] [PubMed] [Google Scholar]
- 24. Shaukat A, Rector TS, Church TR, Lederle FA, Kim AS, Rank JM, et al. Longer withdrawal time is associated with a reduced incidence of interval cancer after screening colonoscopy. Gastroenterology. 2015;149(4):952–57. 10.1053/j.gastro.2015.06.044 [DOI] [PubMed] [Google Scholar]
- 25. de Wijkerslooth TR, Stoop EM, Bossuyt PM, Tytgat KMAJ, Dees J, Mathus‐Vliegen EMH, et al. Differences in proximal serrated polyp detection among endoscopist are associated with variability in withdrawal time. Gastrointest Endosc. 2013;77:617–23. [DOI] [PubMed] [Google Scholar]
- 26. Butterly L, Robinson CM, Anderson J, Weiss JE, Goodrich M, Onega TL, et al. Serrated adenomatous polyp detection increases with longer withdrawal time: results from the New Hampshire colonoscopy registry. Am J Gastroenterol. 2014;109(3):417–26. 10.1038/ajg.2013.442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cavicchi M, Tharsis G, Burtin P, Cattan P, Venezia F, Tordjman G, et al. Difference in physician‐ and patient‐dependent factors contributing to adenoma detection rate and serrated polyp detection rate. Dig Dis Sci. 2019;64(12):3579–88. 10.1007/s10620-019-05808-y [DOI] [PubMed] [Google Scholar]
- 28. Crockett SD, Gourevitch RA, Morris M, Carrell D, Rose S, Shi Z, et al. Endoscopist factors that influence serrated polyp detection: a multicenter study. Endoscopy. 2018;50(10):984–92. 10.1055/a-0597-1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rex DK, Petrini JL, Baron TH, Chak A, Cohen J, Deal SE, et al. Quality indicators for colonoscopy. Gastrointest Endosc. 2006;63(Suppl 4):S16–28. 10.1016/j.gie.2006.02.021 [DOI] [PubMed] [Google Scholar]
- 30. Shaukat A, Kahi CJ, Burke CA, Rabeneck L, Sauer BG, Rex DK. ACG clinical guidelines: colorectal cancer screening 2021. Am J Gastroenterol. 2021;116(3):458–79. 10.14309/ajg.0000000000001122 [DOI] [PubMed] [Google Scholar]
- 31. Hassan C, Quintero E, Dumonceau J.‐M, Regula J, Brandao C, Chaussade S, et al. Post‐polypectomy colonoscopy surveillance: European society of gastrointestinal endoscopy (ESGE) guideline. Endoscopy. 2013;45(10):842–51. 10.1055/s-0033-1344548 [DOI] [PubMed] [Google Scholar]
- 32. Hassan C, Antonelli G, Dumonceau JM, Regula J, Bretthauer M, Chaussade S, et al. Post‐polypectomy colonoscopy surveillance: European society of gastrointestinal endoscopy (ESGE) guidelines – update 2020. Endoscopy. 2020;52:1–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Supporting Information S2
Supporting Information S3
Data Availability Statement
Research data are not shared.


