Figure 3.
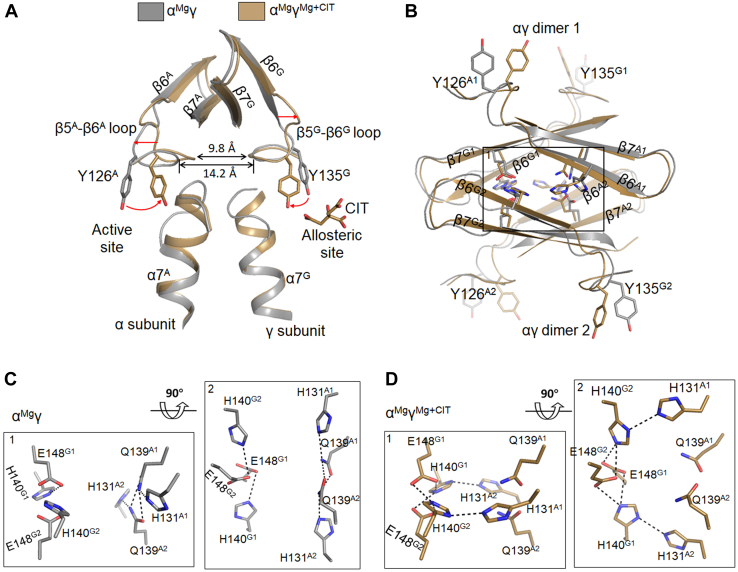
The WT αγ heterodimer shows distinct structural features in the active and inactive states.A, comparison of αMgγ (PDB 5GRH) in the inactive state and αMgγMg+CIT (PDB 5GRI) in the active state shows that the α7 helices and β5-β6 loops assume different conformations. Structural elements of the α and γ subunits are superscripted as “A” and “G,” respectively. Red arrows indicate the conformational changes of the key residues/structure elements upon activation. B, comparison of the αγ-αγ interface in the αMgγ and αMgγMg+CIT structures. Heterodimer 1 and heterodimer 2 are related by crystallographic 2-fold axis and form a heterotetramer via the clasp domains. Structure elements of the αγ heterodimers 1 and 2 are superscripted as “A1” and “G1” and “A2” and “G2”, respectively. Key residues involved in the rearrangement of hydrogen-bonding network are shown in stick model. C, zoom-in views of the hydrogen-bonding network at the αγ-αγ interface in the inactive αMgγ structure. D, zoom-in views of the hydrogen-bonding network at the αγ-αγ interface in the active αMgγMg+CIT structure. Hydrogen bonds are indicated by black dash lines. PDB, Protein Data Bank.