Abstract
Industrial effluents containing trace metals can contaminate water, soil and plants, as well as can cause serious human health impacts. The aim of this study was to assess the concentration of chromium in the soil and khat leaves using spectroscopic methods and to evaluate its potential human health risk on the consumers. The average concentrations of total chromium in the soil samples ranged from 71.01 ± 12.05 to 317.55 ± 23.14 mg kg−1. These values were greater than the control (7.6 ± 0.47 mg kg−1). The average concentrations of total Cr in the khat leaves ranged of 6.5 ± 1.76 to 30.01 ± 2.91 (mg kg−1). These values were higher than the maximum permissible Limits in vegetables 2.3 mg kg−1. The estimated daily intake (EDI) of an adult man weighing 70 kg and consuming on average 100 g of khat leaves per day was found in the range of 0.200–0.454 mg kg−1 body weight day−1. The target hazard quotient (THQ) of Cr from khat leaves consumption was found in the range of 0.001–0.076, which were <1, indicating no potential non-carcinogenic harmful health risk to the consumers in the society. Despite this, the regional EPA must pay close attention to controlling the use of irrigation water contaminated with tannery effluents in order to safeguard khat consumers. Furthermore, consumers must be aware of the health risks and refrain from consuming khat leaves cultivated in the study areas.
Keywords: Chromium, Khat leaves, Bioconcentration factor, Estimated daily intake, Target hazard quotient
Chromium; Khat leaves; Bioconcentration factor; Estimated daily intake; Target hazard quotient.
1. Introduction
Chromium (Cr) is a transition metal found in group VI B in the modern periodic table of the elements. It has a wide range of applications in both elemental and compound forms. In its elemental form, it is primarily used to make steel and alloys, i.e. to increase the metal strength and resistance to corrosion. Cr compounds are used for chrome plating, dye and pigment production, leather tanning agents, catalysis, wood treatment, foundry and laboratory chemical synthesis (Lunk, 2015).
The various industries that use Cr for different chemical processes generate a significant amount of Cr containing waste in to the environment. Cr may enter into the food chain through vegetation in dumping areas and plants irrigated by the untreated/semi treated wastewater from the tanning industries (Khan et al., 2013; Ugulu et al., 2021). Cr occurs in the environment mainly as Cr (III) and Cr (VI) oxidation states. Both chromium ions are interconvertible under favorable environmental conditions (pH, total Cr concentration, presence of reducing and oxidizing compounds, etc.) (Bokare and Choi, 2011). Cr (VI) is extremely toxic than Cr (III) and can cause cancer, damage liver, kidney, and skin of human beings (ATSDR, 2012). Cr (III) is essential in trace amounts for normal protein, fat, and carbohydrate metabolism (Zafra-Stone et al., 2007). It also helps for converting glucose into energy, encouraging healthy blood glucose and blood pressure levels.
Tanneries are pollution-intensive industries generating large amounts of complex liquid and solid wastes into the environment (Alemu et al., 2021; Verma and Sharma, 2022). Typically, Cr salts are used as tanning agents, with chromium sulfate accounting for about 90% of the tanning industry. Out of the total Cr applied in the tanning process, 30–40% remains as waste (Neelam et al., 2018). Most developing countries discharge tannery wastewater with little or no treatment into the water bodies & open lands (Hasnat et al., 2013). This is a common problem in Ethiopia. Studies indicate that Cr pollution in the water and sediments is increasing and becoming one of the main environmental concerns (Wosnie and Wondie, 2014; Alemu and Gabbiye, 2017).
Khat (Catha edulis Forsk) is a flowering shrub with evergreen leaves that by many people chew for its stimulating effect (Woldamanuel, 2019). It grows well in parts of South Western Arabian Peninsula and East Africa (Odenwald et al., 2021). Khat producers in Africa include Ethiopia, Eretria, Somalia, Kenya, Zambia, Rwanda and South Africa. Beyond Africa, it is also produced in Arabian Peninsula, Yemen, Afghanistan, and Sir Lanka (Tadesse and Kebede, 2015). Khat is a source of income in these countries. Khat chewing is popular in some parts of Ethiopia, including Harar, Jimma, and Wlkite. It is also practiced in other parts of the country, such as Bahir Dar, Gondar, Debre Markos etc. Users chew approximately 100–500 g of khat leaves per day depending on its type and availability in the market (Gibbons and Arunotayanun, 2013). The khat plantation and a bundle of khat leaves sold in the market are shown in Figure 1(a) and (b) below.
Figure 1.
Khat plantation (a) and a bundle of khat leaves sold for consumption.
Bahir Dar is one of the most beautiful cities in Ethiopia, which is established on the shores of Lake Tana and Abbay River. It is a hub of commercial and some industrial activities. The majority of industries lack appropriate wastewater treatment plants. Tanneries generated a large amount of Cr containing wastewater, which can be absorbed and accumulated in different parts of the khat plant species. As a result, Cr can enter the food chain and pose a risk of causing serious human health problems such as cancer (Hossain et al., 2017). Plants can absorb Cr from tannery waste via essential ion carriers such as sulfate (Kim et al., 2006). Cr uptake, accumulation, and translocation, depend on its speciation. Moreover, it also varies from one plant species to another (Ertani et al., 2017; Alemu et al., 2020).
The main objective of this study was to determine the concentration of Cr both in the soil (field) and khat leaves, which were irrigated from a small water canal that sometimes mixed with the effluents generated from the nearby tannery industries. Moreover, it investigated the bioconcentration factor of Cr in the khat leaves and the potential health risks on the khat consumers in the society.
2. Materials and methods
2.1. Description of the study area
The study area is found close to Bahir Dar City. The city is located in the northwest of Ethiopia at about 11°36′0″ N latitude and 37°24′0″ E longitude at an elevation of 1800 m. It has warm climate in the winter and high rainfall in the summer (June 25–September 25). The study area covers a water canal used by local farmers to irrigate khat plants as well as vegetables and fruits. The canal runs under the drainage lines from two tannery industries and there are some occasions that the tannery wastewater mixes with the water canal.
2.2. Sampling sites
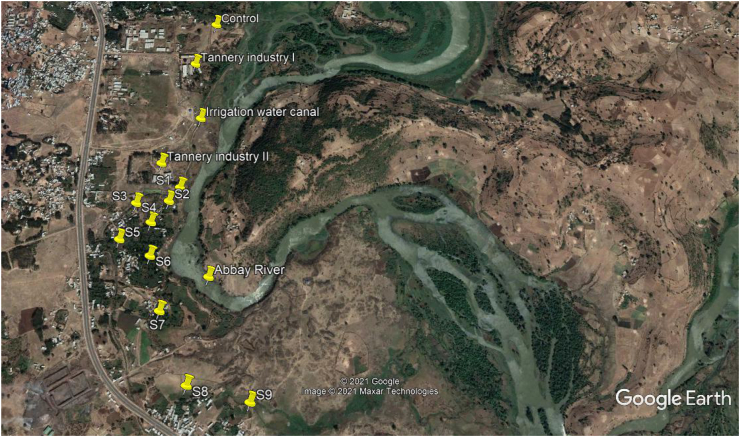
Khat leaves and soil samples were collected randomly in the farm lands from 9 sites (S1–S9) along the water canal of khat plantation (Figure 2). The control site (C) was chosen above the tannery industries in the upstream of Abbay River. The sampling sites were chosen randomly to assess the level of contamination of Cr both in the Khat leaves and soil samples. S1 is found at about 0.5 km from the second tannery and S9 is found close to Sevatamit, which is a satellite city of Bahir Dar.
Figure 2.
Location of sampling sites from S1 to S9 along the water canal (Google Earth).
2.3. Reagents and instruments
All chemicals and reagents used were of analytical grade. KCl (99.9%, Fisher Scientific), 1, 5-diphenylcarbazide (assay ≥99%, Merck), acetone (assay 99.5%, Royal chemicals), Na2CO3 (99.5%, Krishna Chemicals), KH2PO4 (99%, Merck), HNO3 (assay 68–70%) and H2O2 (30%) from BDH laboratory supplies.
2.4. Sample collection and preservation
Soil samples were collected in khat agricultural areas at about 5 cm depth below the surface by composite sampling system using clean polyethylene plastic bags. The samples were air-dried, ground using mortar and pistil and sieved through 250 μm mesh. It was then stored at 4 °C for digestion and analysis.
Khat leave samples were collected from the Khat plant farmlands that were identical to soil sampling sites. The khat leaf samples were cut and collected in a clean plastic bag. It was dried in an open air for one week and kept in an oven at 105 °C overnight. The dried khat leaves were powdered with mortar and pistil, and sieved through 250 μm diameter mesh. Finally, the powdered khat leaves were kept in a cleaned and labeled airtight Ziploc plastic bags for digestion and further analysis. of Cr.
2.5. Analysis of soil and plant samples
2.5.1. Determination of soil pH
The pH of the soil samples was determined by mixing 10 g of dry weight soil with 25 mL of 1M KCl solution in the ratio of 1:2.5 (Kabala et al., 2016). It was stirred for 30 min, and the suspensions were measured with a pH meter (Hanna, HI8424).
2.5.2. Determination of Cr (VI) and total Cr in the soil
The total Cr in the soil was determined by mixing 1 g of dry weight soil with 10 mL of 1:1 nitric acid in 250 mL flask using standard procedures (USEPA, 1996; Ugulu et al., 2021). The flask was covered with watch glass during digestion. It was heated to 95 ± 5 °C and then refluxed for 15 min. 5 mL of concentrated nitric acid was added and refluxed for 30 min. This procedure was repeated until the brown fumes in the sample were completely removed. 3 mL of 30% H2O2 and 2 mL of water were added to the flask and heated in the above temperature ranges until no effervescence was observed. The digestate was diluted and filtered using whatman filter paper. Finally, it was measured by using ICP-OES (Perkin Elmer Optima 8000).
Cr can exist in the soil in the form of Cr (VI). Extraction of Cr (VI) in the soil was done by following the procedure used by different researchers (Scancar et al., 2007; Lesniewska et al., 2017). 5 g of air-dried soil were transferred to 50 mL 0.005 M KH2PO4 and 0.05 M K2HPO4, mixed using rotary shaker (Mcl-300) at 200 rpm for 24 h. The mixture was filtered through whatman filter paper grade 42. The soil sample extracts were analyzed using PerkinElmer Lambda 3000 spectrophotometer with 1.5 Diphenylcarbazide at 540 nm (APHA, 1998).
2.5.3. Determination of total Cr in Khat leaves
0.5 g of ground khat sieved in a 250 μm, 6 mL conc. HNO3, and 2 mL H2O2 were placed in a Teflon tube and closed tightly, digest for 30 min in a microwave digester at temperature of 200 °C and power 1200 w (Wu et al., 1997). It was cooled, filtered and transferred to 50 mL volumetric flask and diluted to the mark with distilled water. In deed the total Cr was determined with ICP-OES (PerkinElmer Optima 8000).
2.6. Bioconcentration factor (BCF)
BCF is the ratio of Cr concentration in edible part of khat leave to Cr concentration in the soil sample (Alemu et al., 2020). The transfer of Cr from the soil to the plant leaves is calculated by Eq. (1).
| (1) |
where Cplant is the Cr concentration in the khat leave and Csoil is the Cr concentration in the soil.
2.7. Estimated daily intake (EDI) and target hazard quotient (THQ)
2.7.1. Estimated daily intake (EDI) of Cr
The estimated daily intake (EDI) of Cr in the khat leaves was determined using Eq. (2) (Chary et al., 2008).
| (2) |
where: is the Cr concentration in khat leaves (mg kg−1 dry weight); is ingestion rate, which is taken as 0.1 kg day−1 (the smallest ingestion rate) for khat fresh leaves (Gibbons and Arunotayanun, 2013); is the average body weight of an adult person which is taken as 70 kg.
2.7.2. Target hazard quotient (THQ)
The health risks of consumers from khat chewing irrigated with contaminated tannery wastewater was calculated using THQs Eqs. (2) and (3) (FAO/WHO, 2011). The non-carcinogenic health risks of khat consumers can be calculated using Eq. (3).
| (3) |
where Ef is exposure frequency (365 day/year); ED is the exposure duration (65 years), FIR is the average consumption of khat (100 g/person/day), CM is metal concentration (mg/kg dry weight); Cf is concentration conversion factor for fresh khat weight to dry weight (0.25) (Staven et al. 2003). is the oral reference dose for the metal (mg kg−1 of body weight per day), Cr (1.5 mg kg−1 per day) (USEPA, 2015). BW is reference body weight for an adult, which is 70 kg. TA is the average exposure time (65 years × 365 days/years).
2.8. Percent of recovery (% R)
Percent of recovery was determined by adding known concentration of Cr to the soil and khat leave samples during the process of digestion. The concentrations of total Cr were analyzed for the spiked and unspiked samples. % R was calculated using Eq. (4) (APHA, 1998):
| (4) |
2.9. Statistical data analysis
Microsoft Excel and Origin lab software were used to analyze the collected data. One-way ANOVA at 95% confidence interval (p < 0.05) was used to determine the significant difference in mean concentration of total Cr in the soil and khat leaves in the sampling sites. Statistical analyses were done using SPSS Statistics 24.0.
3. Results and discussion
3.1. Quality control
The quality of data was controlled by using of standard procedures, calibrating with standard solutions, analysing of reagent blanks, and calculating the percent of recovery. The analyses were done in triplicate, and the average values were expressed as the mean ± SD. The calibration curve correlation coefficients (R) for the analysis of Cr in the soil and khat leaves using ICP-OES and UV-Vis spectroscopy were in the range of 0.989–0.999. The percentage of recovery tests for the soil and khat leaves were found 90% and 87%, respectively. This value falls within the acceptable range of 85–115% (USEPA, 1994).
3.2. pH of the soil samples
The pH of the soil samples was found in the range of 4.84 ± 0.18 to 6.1 ± 0.42 as shown in Table 1. The lowest pH value was shown at site S6 and S8. This suggests that the two sites have a high negative charge on the sediment/soil component (Jordao et al., 1997). Cr occurs in a stable form as Cr (III) under reduced conditions in the pH ranges of 4–8. Cr (III) oxidizes to Cr (VI) and vice versa in the presence of oxidizing and reducing agents, respectively (Avudainayagam et al., 2003). The acidic nature of the soil might be due to use of acidic salts in the processing of leather during the time of sampling. Cr (VI) occurs in an anionic form in most natural environments, predominantly as hydrogen chromate (HCrO4−) between pH 1 and 6. According to USDA (1993) Natural Resources Conservation Service, soil pH ranges roughly from acidic (pH 3.5) to very strongly alkaline (pH 9.0). Soil pH is an important indicator of soil health. It affects crop yields, crop suitability, plant nutrient availability, and soil micro-organism activity, influencing key soil processes. In this study, the average pH values were 5.48, which is in the range of USDA standard (Alemu and Gabbiye, 2017). This value was also equivalent to the control pH value (5.45 ± 0.05).
Table 1.
pH of the soil samples.
| Sample Code | Average pH |
|---|---|
| C | 5.45 ± 0.05 |
| S1 | 5.3 ± 0.07 |
| S2 | 5.3 ± 0.07 |
| S3 | 5.64 ± 0. 13 |
| S4 | 5.78 ± 0.06 |
| S5 | 5.7 ± 0.85 |
| S6 | 4.84 ± 0.35 |
| S7 | 5.76 ± 0.26 |
| S8 | 4.84 ± 0.18 |
| S9 | 6.1 ± 0.42 |
3.3. Level of Cr in the soil
The concentrations of Cr (VI) and total Cr in the soil samples collected are shown in Table 2 below. The level of Cr (VI) contamination of the soil samples was found in the range of 0.03 ± 0.01 to 0.1 ± 0.04 mg kg−1. Higher total Cr concentrations were observed at site S9 (317.55 ± 23.14 mg kg−1) and S8 (278.85 ± 15.13 mg kg−1). The lowest value of total Cr was observed at S4 (71.01 ± 12.05 mg kg−1). All of the Cr (VI) and total Cr values were higher than the control and lower than the Ethiopian Environmental Quality Standards for soil (EEPA, 2003).
Table 2.
Cr (VI) and Cr total concentration in the soil samples.
| Sample code | Cr (VI) (mg kg−1) | Total Cr (mg kg−1) |
|---|---|---|
| C | ND | 7.6 ± 0.47 |
| S1 | 0.07 ± 0.05 | 157.31 ± 11.01 |
| S2 | 0.07 ± 0.02 | 140.12 ± 8.13 |
| S3 | 0.1 ± 0.04 | 186.68 ± 5.03 |
| S4 | 0.04 ± 0.01 | 71.01 ± 12.05 |
| S5 | 0.03 ± 0.01 | 128.67 ± 15.13 |
| S6 | 0.05 ± 0.04 | 224.31 ± 13.39 |
| S7 | 0.07 ± 0.03 | 182.54 ± 9.17 |
| S8 | 0.07 ± o.o5 | 278.85 ± 15.13 |
| S9 | 0.04 ± 0.04 | 317.55 ± 23.14 |
ND – Not detected.
According to single factor ANOVA, the average total Cr concentrations in the soil samples show significant variations, P < 0.05 at the sampling sites. This might be due to discharge of Cr containing wastewater from tannery industries found in the vicinity of the channel. The results were compared with other studies. In Kasur District (Pakistan), the Cr concentration in the soil was found up to 839 mg kg−1 from the vicinity of a tanning effluent and 1829 mg kg−1 in the area of old stagnant pool, which are much higher than the safe limits (Khan et al., 2013).
In Ethiopia, the levels of total Cr and Cr (VI) accumulation in the agricultural soil found in the range of 254.25–1581.66 and 1.17–2.23 mg/kg around the Ethiopia Tannery Share Company, respectively (Homa et al., 2016). Reda (2015) also reported a total Cr level of 2017.24 mg/kg in the surrounding soil of Modjo Tannery effluent. A study of Cr pollution of the soil in the riparian of Abbay River from tannery and textile industries indicated a maximum of an average value of 232.465 ± 56.219 mg/kg (Alemu and Gabbiye, 2017). The increased concentration of Cr in the soil can lead to an increase in the uptake and accumulation of Cr in the khat leaves that can affect the heath of the consumers.
3.4. Total Cr in khat (Catha edulis Forsk) leaves
The average concentrations of total Cr in khat leave were found in the range of 6.5 ± 1.76 to 35.71 ± 0.98 (mg kg−1). The highest total Cr concentration (35.71 ± 0.98 mg kg−1) was observed at S2 and the lowest total Cr (6.5 ± 1.76) was observed at PS4 (Table 3). There was a statistically significant difference (P < 0.05) in the mean concentration of total Cr in the khat leaves taken at different sampling sites. This might be variation in the distances of the sampling sites from the irrigation canal contaminated with the tannery wastewater. The total Cr concentrations in the khat leaves were higher than the maximum permissible Limits in vegetables, 2.3 mg kg−1 (FAO/WHO Codex Alimentarius Commission, 2001).
Table 3.
Total Cr in the Khat leaves.
| Sample ID | Total Cr (mg kg−1) |
|---|---|
| C | 7.50 |
| KS1 | 31 ± 1.32 |
| KS2 | 35.71 ± 0.98 |
| KS3 | 16 ± 1.01 |
| KS4 | 6.5 ± 1.76 |
| KS5 | 13 ± 0.87 |
| KS6 | 28.08 ± 2.79 |
| KS7 | 30.01 ± 2.91 |
| KS8 | 27.75 ± 4.85 |
| KS9 | 29.02 ± 3.22 |
NB: KS means Khat plant at sampling site
This study was compared to other similar studies in Eastern Ethiopia vegetables including khat cultivated by irrigating with sewage wastewater. The total Cr concentration in the khat leaves was found in the range of 9.04–15.54 mg kg−1 (Deribachew et al., 2015). Another study in the southern region of Ethiopia indicated that the total Cr (mg kg−1) in the khat leaves in Koyra, Didole, and Konso were 1.52 ± 0.13, 2.75 ± 0.32, and 4.09 ± 0.29 respectively (Desta and Ataklti, 2015). Maleki and Zarasvand (2008) reported 7.9 mg kg−1 of total Cr in vegetable samples.
3.5. Bioconcentration factor (BCF)
BCF provides quantitative information for assessment the risk of Cr from khat chewing to human health. BCF helps to estimate the plants potential to accumulate Cr in its tissue, especially in the edible part of the leaves of khat plant. The BCF value was found in the range of 0.086–0.255 at all sampling sites (Table 4). The calculated BCF values are less than one, which indicates the khat plant accumulates less Cr in its leaves (Baker, 1981). The BCF of Cr in tomato and cabbage at Koka and Ejersa, Ethiopia indicated 0.018 and 0.090, respectively (Bayissa and Gebeyehu, 2021). The BCF of Cr in the fruits of medicinal plants was found in the range of 0.3–0.439 (Parveen et al., 2020). Chang et al. (2014) also reported that the BCF of Cr in six leaf vegetables in the range of 0.0002–0.027. The BCF of Cr in this study was higher due to irrigation of contaminated tannery wastewater to the khat plants.
Table 4.
Chromium BCF in the khat leaves.
| Sample code | BCF |
|---|---|
| C | 0.197 |
| S1 | 0.197 |
| S2 | 0.255 |
| S3 | 0.086 |
| S4 | 0.092 |
| S5 | 0.101 |
| S6 | 0.125 |
| S7 | 0.164 |
| S8 | 0.099 |
| S9 | 0.091 |
3.6. Estimated daily intake (EDI) and target hazard quotient (THQ)
3.6.1. Estimated daily intake (EDI)
The daily intake of Cr depends on its concentration in the khat leaves and its daily consumption of khat leaves. The effect Cr on humans can be influenced by an individual’s body weight. The EDI of an adult man with 70 kg weight who consumes on average 100 g of khat leaves per day in the study area was calculated and found in the range of 0.200–0.454 mg/kg body weight/day (Table 5). This value (EDI of Cr) will be increased for people with lower body weight and frequently consuming khat. The EDI of the control is 0.011 mg/kg body weight/day, which is very low compared to other sampling sites. The EDI of Cr for an adult person consuming 240 g/day of tomato and cabbage in Koka area, Ethiopia reported values of 2.76 × 10−4 and 1.52 × 10−3 mg/day/kg body weight, respectively (Bayissa and Gebeyehu, 2021). The daily intake of Cr in khat leaves was higher than the total intake from food (0.008 mg/day) (Shaheen et al., 2016).
Table 5.
Calculated EDI and THQ values of Cr via chewing of khat.
| Sample code | EDI (mg/kg. day) | THQ |
|---|---|---|
| C | 0.011 | 0.002 |
| S1 | 0.225 | 0.038 |
| S2 | 0.200 | 0.034 |
| S3 | 0.267 | 0.045 |
| S4 | 0.102 | 0.001 |
| S5 | 0.184 | 0.031 |
| S6 | 0.321 | 0.054 |
| S7 | 0.261 | 0.044 |
| S8 | 0.399 | 0.067 |
| S9 | 0.454 | 0.076 |
3.6.2. Target hazard quotient (THQ)
The THQ of total Cr from the consumption of khat leaves in the study area found in the range of 0.001 (S4) to 0.076 (S9) (Table 5). In the study area, the calculated THQ values of Cr were less than 1.0. According to USEPA (2015), THQ >1 indicates a higher possibility to occur for non-cancer health disorders. The THQ values indicated that the level of Cr in the sampling sites were within the tolerable limit of non-carcinogenic harmful health risk in the study area. This doesn’t mean that all the consumers are safe from the risk of Cr gained through khat chewing. The THQ value can be varied based on the dose of khat leave consumption and body weight of the consumer.
4. Conclusions
This study found Cr concentrations in the soil and khat leaves grown using irrigation water contaminated with tannery wastewater and consumed by many people in Bahir Dar and the surrounding area. Furthermore, BCF, EDI and THQ health risk implications of Cr from chewing of khat leaves were investigated. The total Cr concentrations were found in the average range of 71.01 ± 12.05 to 317.55 ± 23.14 mg/kg in the soil and 6.5 ± 1.76 to 35.71 ± 0.98 mg/kg in the khat leaves. The total Cr concentrations in the khat leaves exceeded the maximum permissible Limit in vegetables (i.e. 2.3 mg kg−1). The EDI of Cr from consumption of khat leaves for an adult man (70 kg) on average was 0.268 mg/day. Its risk on human health was evaluated with THQ and it was less than 1. This indicates no potential non-carcinogenic harmful health risks to the consumers.
5. Recommendations
Despite the THQ < 1, this doesn’t mean safe. This value depends on the frequency of khat consumption and body weight of the consumer. Therefore, to reduce the health risk on the khat consumers, appropriate binding reclamations has to be developed to solve the challenges of land contamination with Cr from tannery effluents.
Declarations
Author contribution statement
Agegnehu Alemu: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Alemwork Mekonnen: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Agency for Toxic Substances and Disease Registry, ATSDR . Department of Health and Human Services, Public Health Services; Atlanta, GA, U. S: 2012. Toxicological Profile for Chromium.http://www.atsdr.cdc.gov [PubMed] [Google Scholar]
- Alemu A., Gabbiye N. Assessment of chromium contamination in the surface water and soil at the riparian of Abbay River caused by the nearby industries in Bahir Dar city, Ethiopia. Water Pract. Technol. 2017;12:72–79. [Google Scholar]
- Alemu A., Gabbiye N., Lemma B. Application of integrated local plant species and vesicular basalt rock for the treatment of chromium in tannery wastewater in a horizontal subsurface flow wetland system. J. Environ. Chem. 2020;8 [Google Scholar]
- Alemu A., Gabbiye N., Lemma B. Evaluation of tannery wastewater treatment by integrating vesicular basalt with local plant species in a constructed wetland system. Front. Environ. Sci. 2021;9 [Google Scholar]
- APHA . twentieth ed. American Public Health Association Inc Washington DC; USA: 1998. Standard Methods for Examination Water and Wastewater.https://trove.nla.gov.au/work/16646325 [Google Scholar]
- Avudainayagam S., Megharaj M., Owens G., Kookana R.S., Chittleborough D., Naidu R. Chemistry of chromium in soils with emphasis on tannery waste sites. Rev. Environ. Contam. Toxicol. 2003:53–91. doi: 10.1007/0-387-21728-2_3. Springer, New York, NY. [DOI] [PubMed] [Google Scholar]
- Baker A.J. Accumulators and excluders-strategies in the response of plants to heavy metals. J. Plant Nutr. 1981;3:643–654. [Google Scholar]
- Bayissa L.D., Gebeyehu H.R. Vegetables contamination by heavy metals and associated health risk to the population in Koka area of central Ethiopia. PLoS One. 2021;16 doi: 10.1371/journal.pone.0254236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokare A.D., Choi W. Advanced oxidation process based on the Cr (III)/Cr (VI) redox cycle. Environ. Sci. Technol. 2011;45:9332–9338. doi: 10.1021/es2021704. [DOI] [PubMed] [Google Scholar]
- Chang C.Y., Yu H.Y., Chen J.J., Li F.B., Zhang H.H., Liu C.P. Accumulation of heavy metals in leaf vegetables from agricultural soils and associated potential health risks in the Pearl River Delta, South China. Environ. Monit. Assess. 2014;186:1547–1560. doi: 10.1007/s10661-013-3472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chary N.S., Kamala C.T., Raj D.S.S. Assessing risk of heavy metals from consuming food grown on sewage irrigated soils and food chain transfer. Ecotoxicol. Environ. Saf. 2008;69:513–524. doi: 10.1016/j.ecoenv.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Deribachew B., Amde M., Nigussie-Dechassa R., Taddese A.M. Selected heavy metals in some vegetables produced through wastewater irrigation and their toxicological implications in Eastern Ethiopia. Afr. J. Food Nutr. Sci. 2015;15:10013–10032. [Google Scholar]
- Desta A., Ataklti A. Profile of essential and non-essential metals in soil and in khat (catha edulis forsk) leaves cultivated in southern region, Ethiopia. Am. J. Phys. Chem. 2015;4:58–64. [Google Scholar]
- EEPA . Ethiopian Environmental Protection Authority of Ethiopia (EPA) and United Nations Industrial Development Organization, UNIDO; Addis Ababa: 2003. Guideline Ambient Environment Standards for Ethiopia. [Google Scholar]
- Ertani A., Mietto A., Borin M., Nardi S. Chromium in agricultural soils and crops: a review. Water Air Soil Pollut. 2017;228:190. [Google Scholar]
- FAO/WHO Codex Alimentarius Commission . 2001. Food Additives and Contaminants; pp. 1–289. (Joint FAO/ WHO Food Standards Programme; ALINORM 01/12A). [Google Scholar]
- FAO/WHO . 2011. oint FAO/WHO Food Standards Programme Codex Committee on Contaminants in Foods, Food CF/5 INF/1. Fifth Session. The Hague, The Netherlands.ftp://ftp.fao.org/codex/meetings/CCCF/cccf5/cf05_INF.pdf J. [Google Scholar]
- Gibbons S., Arunotayanun W. 2013. Natural Product Fungal and Herbal. (U: Novel psychoactive substances. Dargan P, Wood D). [Google Scholar]
- Hasnat A., Rahman I., Pasha M. Assessment of environmental impact for tannery industries in Bangladesh. Int. J. Environ. Sustain Dev. 2013;4:217. [Google Scholar]
- Homa D., Haile E., Washe A.P. Determination of spatial chromium contamination of the environment around industrial zones. Int. J. Anal. Chem. 2016;2016 doi: 10.1155/2016/7214932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain M.S., Roy P., Islam M., Chowdhury M.A.Z., Fardous Z., Rahman M.A., Saifullah A.S.M., Hasan M., Rahman M.M. Retracted human health risk of chromium intake from consumption of poultry meat and eggs in Dhaka, Bangladesh. J. Health Pollut. 2017;7:30–36. doi: 10.5696/2156-9614-7.14.30. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Jordao C.P., Pereira J.L., Jham G.N. Chromium contamination in sediment, vegetation and fish caused by tanneries in the State of Minas Gerais, Brazil. Sci. Total Environ. 1997;207:1–11. doi: 10.1016/s0048-9697(97)00232-5. [DOI] [PubMed] [Google Scholar]
- Kabala C., Musztyfaga E., Gałka B., Labuńska D., Manczynska P. Conversion of soil pH 1: 2.5 KCl and 1: 2.5 H2O to 1: 5 H2O: conclusions for soil management, environmental monitoring, and international soil Databases. Pol. J. Environ. Stud. 2016;25:647–653. [Google Scholar]
- Khan S.A., Ibrahim M., Jamil Y., Islam M.S., Abbas F. Spectrochemical analysis of soil around leather tanning industry using laser induced breakdown spectroscopy. J. Chem. 2013;2013 [Google Scholar]
- Kim Y.J., Kim J.H., Lee C.E., Mok Y.G., Choi J.S., Shin H.S., Hwang S. Expression of yeast transcriptional activator MSN1 promotes accumulation of chromium and sulfur by enhancing sulfate transporter level in plants. FEBS Lett. 2006;580:206–210. doi: 10.1016/j.febslet.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Lesniewska B., Gontarska M., Godlewska-Zyłkiewicz B. Selective separation of chromium species from soils by single-step extraction methods: a critical appraisal. Water Air Soil Pollut. 2017;228:274. doi: 10.1007/s11270-017-3459-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunk H.J. Discovery, properties and applications of chromium and its compounds. ChemTexts. 2015;1:1–17. [Google Scholar]
- Maleki A., Zarasvand M.A. Heavy metals in selected edible vegetables and estimation of their daily intake in Sanandaj, Iran. Southeast Asian J. Trop. Med. Publ. Health. 2008;39:335. [PubMed] [Google Scholar]
- Neelam A., Alamgir A., Kanwal H. Determination of chromium in the tannery wastewater, Korangi, Karachi. Int. J. environ. Sci. Nat. Resour. 2018;15:122–125. [Google Scholar]
- Odenwald M., Klein A., Warfa N. Springer; Cham: 2021. Khat addiction; pp. 229–239. (Textbook of Addiction Treatment). [Google Scholar]
- Parveen R., Abbasi A.M., Shaheen N., Shah M.H. Accumulation of selected metals in the fruits of medicinal plants grown in urban environment of Islamabad, Pakistan. Arab. J. Chem. 2020;13:308–317. [Google Scholar]
- Reda A.H. Study on the pollution levels of trace metals from modjo tannery effluent in the surrounding river water and soil. Sci. J. Anal. Chem. 2015;3:56–60. [Google Scholar]
- Scancar J., Zupancic M., Milacic R. Development of analytical procedure for the determination of exchangeable Cr(VI) in soils by anion-exchange fast protein liquid chromatography with electrothermal atomic absorption spectrometry detection. Water Air Soil Pollut. 2007;185:121–129. [Google Scholar]
- Shaheen N., Irfan N.M., Khan I.N., Islam S., Islam M.S., Ahmed M.K. Presence of heavy metals in fruits and vegetables: health risk implications in Bangladesh. Chemosphere. 2016;152:431–438. doi: 10.1016/j.chemosphere.2016.02.060. [DOI] [PubMed] [Google Scholar]
- Staven L.H., Napier B.A., Rhoads K., Strenge D.L. A compendium of transfer factors for agricultural and animal products (No. PNNL-13421) Pacific Northwest National Lab (PNNL); Richland, WA (United States): 2003. [Google Scholar]
- Tadesse S.F., Kebede W.L. Determination of the level of selected heavy metals from khat leaves (catha edulis forsk) grown in Gidolle Konso and Koyira, southern Ethiopia. Sci. J. Anal. Chem. 2015;3:115–121. [Google Scholar]
- Ugulu I., Khan Z.I., Safdar H., Ahmad K., Bashir H. Chromium bioaccumulation by plants and grazing livestock as affected by the application of sewage irrigation water: implications to the food chain and health risk. Int. J. Environ. Res. 2021;15:261–274. [Google Scholar]
- USDA . United States Department of Agriculture; 1993. Soil Survey Manual.http://soils.usda.gov/technical/manual/contents/chapter3.html (accessed 22 July 2020) [Google Scholar]
- USEPA . 1994. Method 200.7: Determination of Metals and Trace. (Elements in Water and Wastes by Inductively Coupled Plasma-Atomic Emission Spectrometry, Revision 4.4). [Google Scholar]
- USEPA . 1996. Method 3050B: Acid Digestion of Sediments, Sludges, and Soils. Revision 2. [Google Scholar]
- USEPA . United States Environmental Protection. Agency; 2015. Risk Based Screening Table-Generic, Summary Table.http://www.epa.gov/risk/riskbased-screening-table-generic-tables URL. Accessed 31.01.2016. [Google Scholar]
- Verma S.K., Sharma P.C. Current trends in solid tannery waste management. Crit. Rev. Biotechnol. 2022:1–18. doi: 10.1080/07388551.2022.2068996. [DOI] [PubMed] [Google Scholar]
- Woldamanuel M.M. Assessment of selected nutrients and toxic chemicals in Ethiopian khat. Science. 2019;7:26–35. [Google Scholar]
- Wosnie A., Wondie A. Assessment of downstream impact of Bahir Dar tannery effluent on the head of Blue Nile River using macroinvertebrates as bioindicators. Int. J. Biodivers. Conserv. 2014;6:342–350. [Google Scholar]
- Wu S., Feng X., Wittmeier A. Microwave digestion of plant and grain reference materials in nitric acid or a mixture of nitric acid and hydrogen peroxide for the determination of multi-elements by inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 1997;12:797–806. [Google Scholar]
- Zafra-Stone S., Bagchi M., Preuss H.G., Bagchi D. 2007. Benefits of chromium (III) complexes in animal and human health; pp. 183–206. (The Nutritional Biochemistry of Chromium (III)). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.