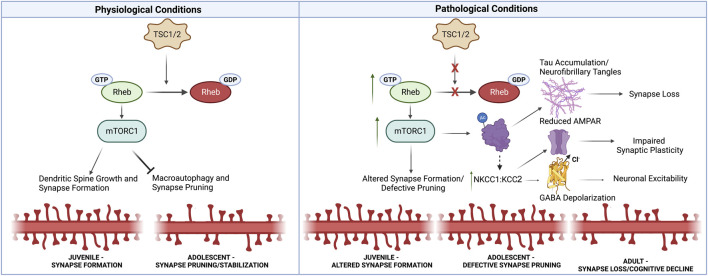
FIGURE 1.
Synaptic Alterations Linking Neurodevelopmental and Neurodegenerative Pathology in TS. Under physiological conditions, TSC1/2 inhibit mTORC1 activity through Rheb-GTPase. mTORC1 promotes synapse formation, but prevents macroautophagy-mediated synaptic pruning later in development. Thus under pathological conditions in TS, mTORC1 activity is increased, resulting in defective synaptic pruning in adolescence. Increased mTORC1 also promotes tau aggregation through acetylation and phosphorylation. Furthermore, impaired autophagy prevents the clearance of tau aggregates, ultimately driving synapse loss. Additionally, TS patients exhibit alterations in NKCC1:KCC2 that resemble immature neural circuits. These changes may in part be driven by tau accumulation. Increased NKCC1:KCC2 can alter GABA polarization at inhibitory synapses, but also impairs AMPAR recruitment at excitatory synapses which is necessary for synaptic plasticity associated with learning and memory. Thus, early developmental synaptic alterations likely lead to the accumulation of neurotoxic tau aggregates, impair synaptic plasticity necessary for learning and memory, and alter neuronal excitability, thus driving synapse loss in neurodegenerative disorders. Created with Biorender.com.