Extended Data Fig. 7. In vivo activity of LA7 and LA9.
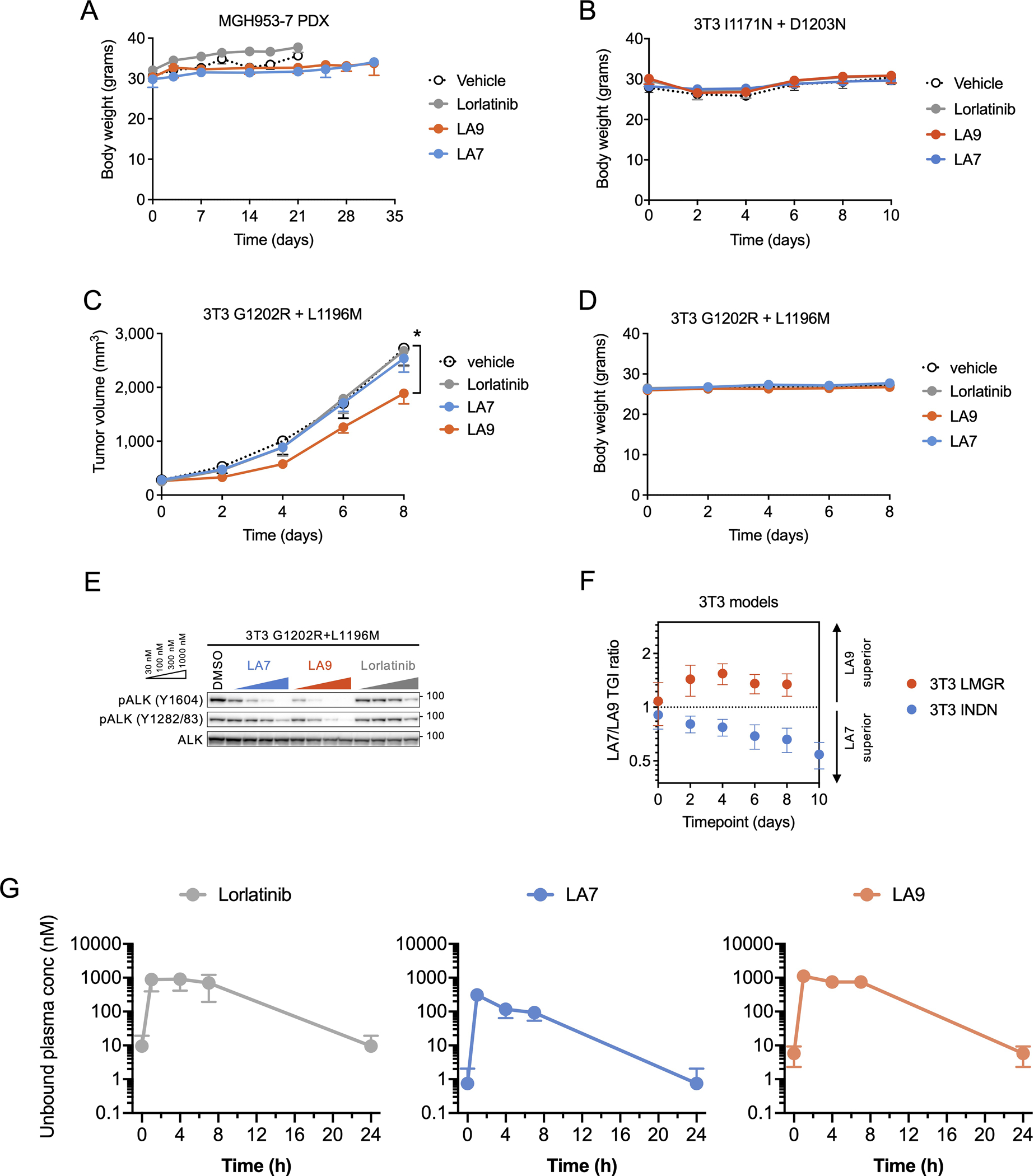
(A) Body weight of mice bearing MGH953-7 during the course of treatment (N = 6 mice per group). (B) Body weight of mice bearing NIH3T3 EML4-ALK I1171N + D1203N xenograft tumors during the course of treatment (N = 6 per group). (C) Change in tumor volume of NIH3T3 G1202R + L1196M xenograft tumors. Mice were treated with lorlatinib 6 mpk QD, LA7 20 mpk QD, or LA9 40 mpk QD (N = 6 mice per group). Tumors treated with LA9 were significantly smaller on day 8 compared to those with vehicle (p = 0.048, two-tailed t-test). (D) Body weight of mice bearing NIH3T3 EML4-ALK I1171N + D1203N xenograft tumors during the course of treatment (N = 6 mice per group). (E) Western blot analysis of NIH3T3 cells expressing ALK G1202R + L1196M and treated with the indicated drugs for 1 hour. (F) The ratio of tumor growth inhibition (TGI) with LA7 and LA9 in NIH3T3 EML4-ALK I1171N + D1203N or G1202R + L1196M xenograft tumors, corresponding to Fig. 5D and Extended Data Fig. 7C. Error bars indicate SEM for 6 mice per group. (G) Unbound systemic concentrations of lorlatinib, LA7 and LA9 were calculated based on the total drug concentrations in whole blood measured in vivo and the unbound fraction in blood (fu,b; calculated as fu,p / BPR). Data are mean and SEM of three mice.
