Extended Data Fig. 9. Relative changes in IC50 values for lorlatinib, LA7 and LA9 against single and compound ALK mutations.
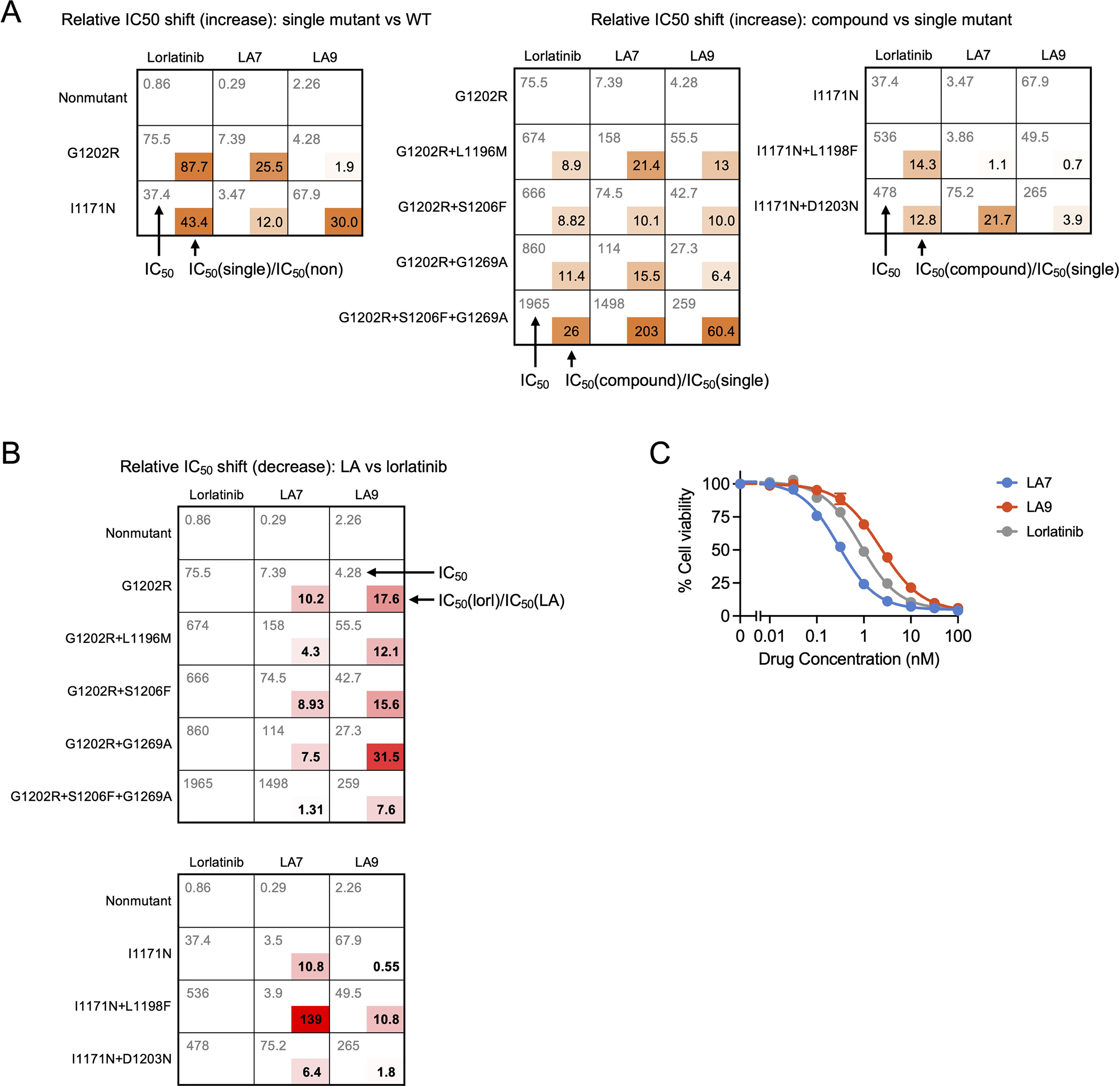
(A) Lorlatinib, LA7 and LA9 cellular IC50 values (upper left) and IC50 ratios (lower right) of single mutant vs nonmutant ALK (left panel) or compound mutant vs single mutant ALK (middle and right panels). IC50 values correspond to data shown in the Source Data. (B) Fold improvement of LA7 or LA9 compared to lorlatinib against single and compound mutations (calculated by dividing the lorlatinib IC50 by the LA IC50). (C) Cell viability assays performed with Ba/F3 cells expressing nonmutant EML4-ALK with 0.01–100 nM lorlatinib, LA7 or LA9 for 48 hours. The viabilities were measured with CellTiter-Glo assay. Data are mean and SEM of three independent experiments.
