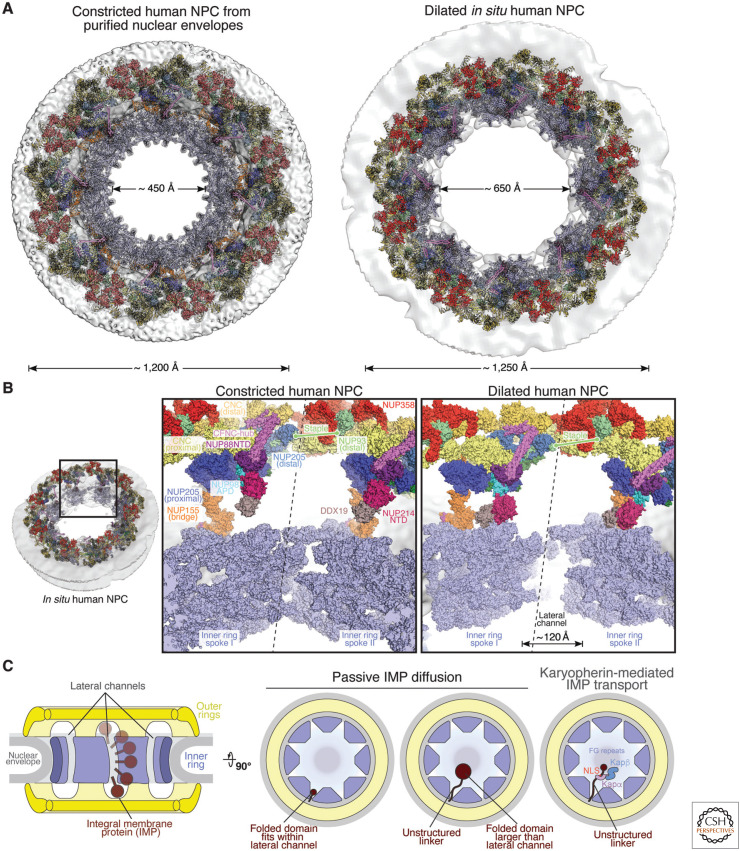
Figure 2.
Dilation of the nuclear pore complex (NPC) and lateral channel formation. (A) Cytoplasmic views of the near-atomic composite structure of constricted and dilated human NPCs (Bley et al. 2022; Mosalaganti et al. 2022). The nuclear envelope is shown as a gray isosurface and nucleoporins are shown in cartoon representation. (B) Close-up views of the interface between two spokes, separated by a dashed line, in the constricted and dilated human NPCs. Nucleoporins are shown in surface representation, with the inner ring spokes uniformly colored in pale blue. (C) Schematic representation of the NPC symmetric core cross section, illustrating inner nuclear membrane integral membrane protein (INM-IMP) passage through the lateral channels. Top views of the NPC symmetric core illustrate that lateral channels can accommodate freely diffusing small, folded pore-facing domains or unstructured linkers tethering larger folded domains or karyopherin-binding nuclear localization sequences (NLSs; classical Kapα/Kapβ-mediated import shown). (Figure adapted from Petrovic et al. 2022, with permission from the authors.)