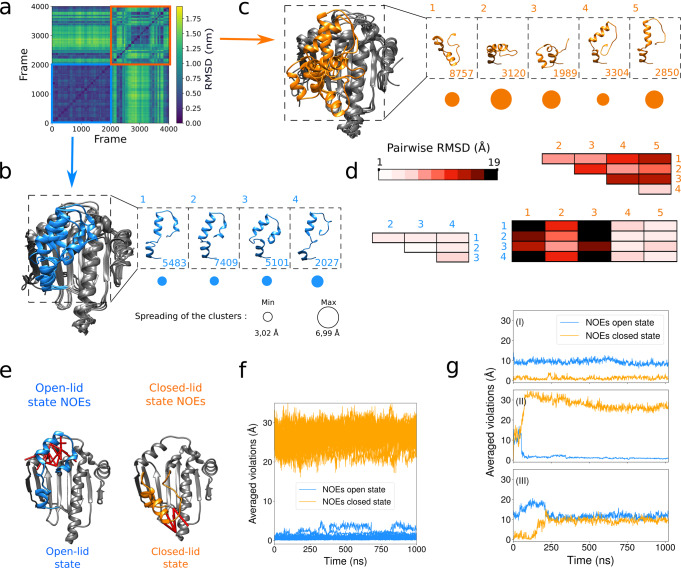
Fig. 4. Molecular dynamic investigation of HSP90α-NTD ATP-lid open and closed states.
a Pairwise RMSD among the 4000 ATP-lid structures obtained in the forty Molecular Dynamics simulations of 1 µs. From blue to yellow, the RMSD values increase. The initial models are the two ensembles of experimentally refined structures (see Fig. 3a, b). The first and second half of the 4000 frames are extracted from simulations starting with the ATP-lid in the open state (blue square) or in the closed state (orange square), respectively. b Superimposition of the 4 centroids representing the 4 clusters describing 20020 conformations extracted from the simulations starting with ATP-lid open-state conformers. The ATP-lid centroids are represented in b.1, b.2, b.3, and b.4 with the total number of members for each cluster. Below, the circle radii are proportional to the spreading of the cluster (in Å). c Same as (b) for the 5 centroids representing the 5 clusters describing 20020 conformations extracted from the simulation starting with ATP-lid closed-state conformers. d Pair-wise Cα-RMSD between the centroids of the clusters represented in (b) and (c), with a color-code that is darker when the RMSD is higher. Blue and orange indices correspond to the clusters presented in (b) and (c) issued from the simulations starting from open and closed states, respectively. e Representation of NOE distance restraints characteristics of ATP-lid open and closed states, on the corresponding structures. f Violations of characteristics NOEs monitored during the molecular dynamics simulations performed without restraints, for the 20 simulations starting with the ATP-lid in the open state (see methods for definition of computed violations). The values are averaged over all the specific distance restraints depicted in (e), either for the ATP-lid open states (blue curves) or for closed states (orange curves) (Supplementary Table 2). g As (f), for three typical simulations starting with the ATP-lid in the closed state: one remains stable (I), one undergoes a transition towards the ATP-lid in the open-state (II), and one derives towards region of conformational space that is neither the closed, nor the open ATP-lid state (III).