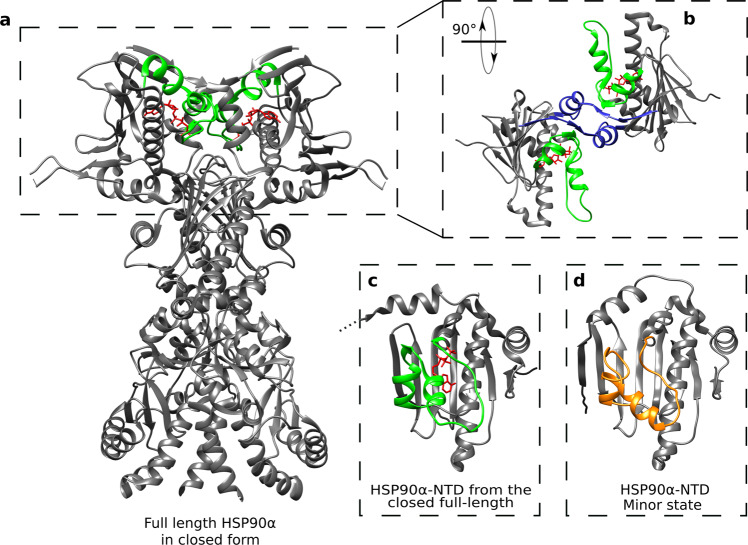
Fig. 6. Structure comparison of HSP90α-NTD excited state with full-length dimeric HSP90 α functional cycle intermediate.
a Structure of the homodimer full-length HSP90α in closed form stabilized by p23, FKBP51 and ATP (PDB 7L7J66). The segment covering the nucleotide-binding site and ATP are colored in green and red, respectively. b Zoom on the two N-terminal domains of the full-length HSP90α, turned by 90°. The blue segments represent the β strands exchanging between the two chains of the homodimer. c N-terminal domain of HSP90α (PDB 7L7J). d Average structure of the calculated ensemble for ATP-lid closed (excited) state of apo HSP90α-NTD. The segment 98–136 is represented in orange.