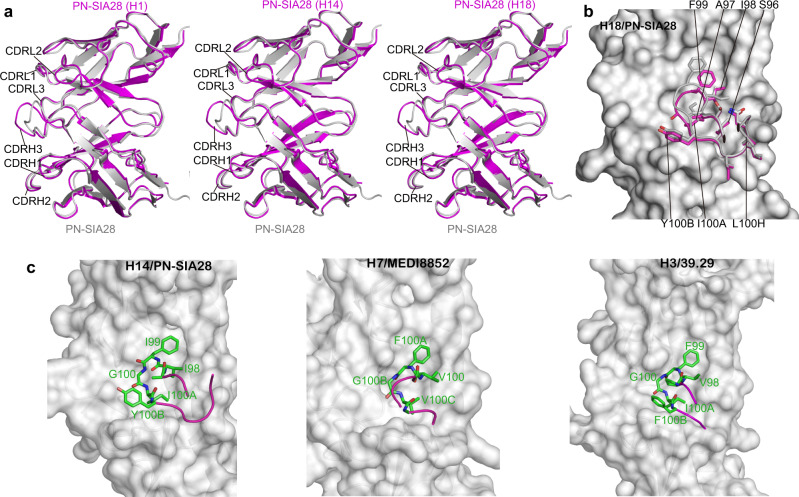
Fig. 6. PN-SIA28 binds to a conserved stem region of HA through CDRH3 conformational rearrangements upon complex formation.
a Overlay of PN-SIA28 bound to H1, H14, or H18 (magenta) and the PN-SIA28 Fab alone (grey). The CDRs of PN-SIA28 are labeled. b Conformational rearrangements in PN-SIA28 upon complex formation. Conformational change of the CDRH3 loops upon HA engagement. The CDRH3 of the apo and bound structures is colored gray and magenta, respectively. The beginning and end of the moving regions are indicated with black ovals. The HA is shown in surface representation. The apo structure does not make interactions with HA and does not fit into its surface features; the conformational change is necessary for productive HA engagement. c Comparison of the structures of the CDRH3 in the complexes between PN-SIA28/H14 (left panel), MEDI8852/H7 (middle panel), and 39.29/H3 HA (right panel). In all cases, the key amino acids are shown in stick representation with other loops of the antibody shown as coils, colored according to (b). The HAs are shown as gray surfaces.