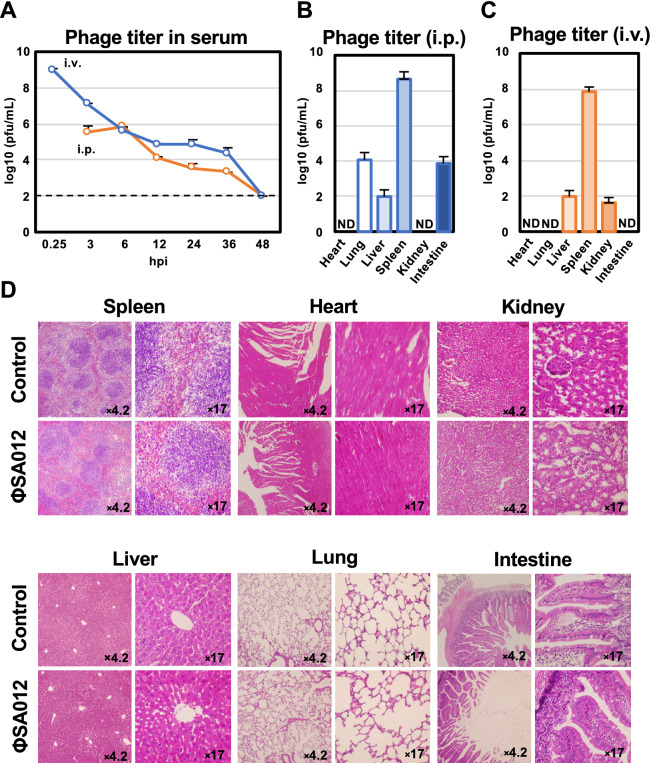
Figure 4.
Pharmacokinetics and side effects of ΦSA012 in mice. (A) Pharmacokinetics of ΦSA012 administered with i.p. and i.v. injections into mice. Phage titers in the serum were monitored from 3 to 48 hpi for i.p. and from 0.25 hpi to 48 hpi for i.v. injections. The titers of ΦSA012 in serum samples are presented as means ± SD (n = 3). 102 pfu/mL is the limit of detection of the phage in the plaque assay. (B) Accumulation of ΦSA012 in organs at 48 hpi after i.p. injection. The titers of ΦSA012 in organ homogenates are presented as means ± SD (n = 3). (C) Accumulation of ΦSA012 in organs at 48 hpi after i.v. injection. The titers of ΦSA012 in organs homogenates are presented as means ± SD (n = 3). ND means that plaques were not detected, indicating values less than 1.2 × 102 pfu/g in the heart, 8.9 × 101 pfu/g in the lung, 4.5 × 102 pfu/g in the kidney, and 8.7 × 101 pfu/g in the intestine. (D) Histopathology and pathological changes of organs in ΦSA012-inoculated mice (i.p.). The spleens, hearts, kidneys, livers, lungs, and intestines were stained with hematoxylin and eosin. The number in each picture represents magnification.