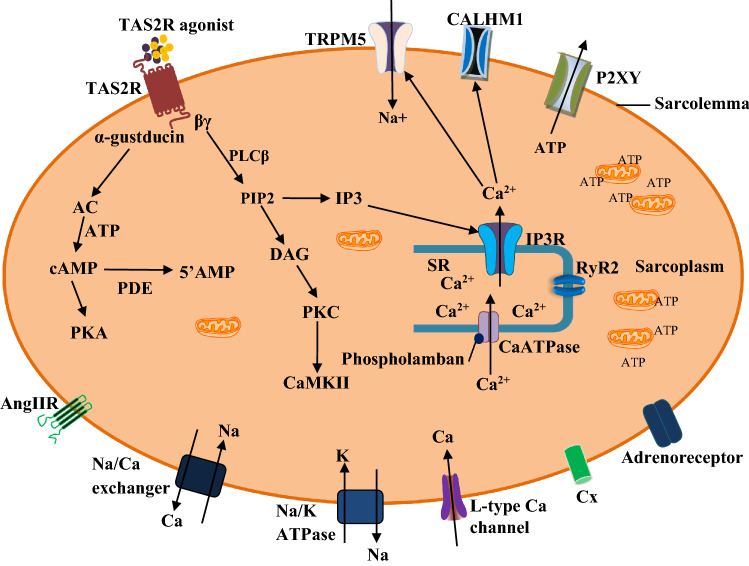
Fig. 1.
Signal transduction mechanisms of cardiac bitter taste receptors (TAS2R). The pathogenic or toxigenic molecules activate TAS2R (Freund et al. 2018) to trigger the dissociation of α-gustducin from the βγ subunit. The former stimulates the membrane bound enzyme, adenylate cyclase (AC). This enzyme produces cyclic adenosine monophosphate (AMP) in the presence of adenosine triphosphate (ATP) (Jeon et al. 2011; Workman et al. 2018; Xie et al. 2018). Cyclic AMP activates some ion channels and intracellular enzymes, including protein kinase A (PKA), which phosphorylates its downstream targets. However, cyclic AMP is hydrolysed by phosphodiesterases (PDE3, -4) to form 5’AMP, thereby decreasing the level of activated PKA (Foster et al. 2014). The βγ subunit stimulates phospholipase Cβ (PLCβ), which mediate the formation of diacylglycerol (DAG) and 1,4,5-inositol trisphosphate (IP3) from phosphatidylinositol 4,5-bisphosphate (PIP2). IP3 stimulates IP3 receptor (IP3R) to mediate cytosolic release of Ca2+. The increase in cytosolic Ca2+ activates transient receptor potential cation channel, subfamily M, member 5 (TRPM5), calcium homeostasis modulator 1 (CALHM1), and ryanodine receptor 2 (RyR2) of the sarcoplasmic reticulum (SR) (Chandrashekar et al. 2000; Jeon et al. 2011; Workman et al. 2018; Xie et al. 2018). Elevation of cytosolic Ca2+ may cause ATP secretion via P2XY channel (Welcome et al. 2015). However, increase in Ca2+ level is only transient and not able to cause contraction of the muscle cell. DAG activates protein kinase C (PKC), which phosphorylates other intracellular proteins and membrane receptors such as Ca2+-calmodulin protein kinase II (CaMKII), angiotensin II receptor (Ang IIR), connexins (Cx) (Manson et al. 2014; Welcome et al. 2015)