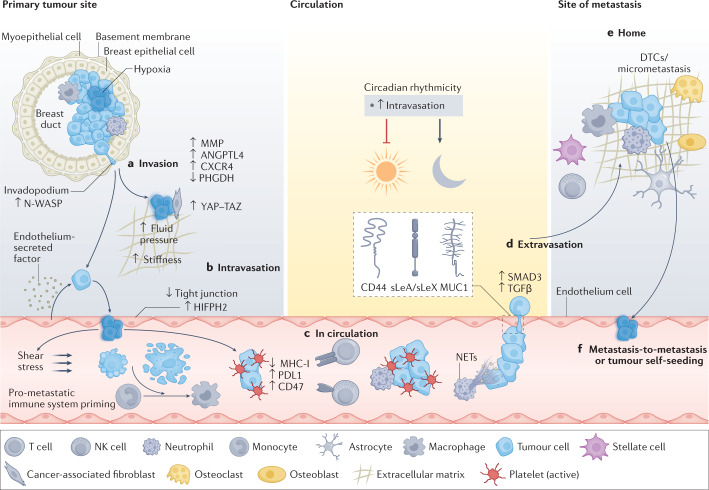
Fig. 1. Stepwise progression of the metastatic cascade.
a, Invasion of cancer cells. The formation of invasive features (for example, invadopodia) and hypoxic conditions (indicated by a blue haze) favour release of cancer cells away from the primary tumour site via upregulation of hypoxia inducible factor 1α (HIF1α), NMYC downstream-regulated gene 1 protein (NDRG1) and vascular endothelial growth factor A (VEGFA). This is further enhanced by metastasis-promoting features, including the expression of CXC-chemokine receptor 4 (CXCR4) and angiopoietin-like protein 4 (ANGPTL-4), the secretion of matrix metalloproteinases (MMPs), decreased expression of phosphoglycerate dehydrogenase (PHGDH) and cytoskeletal rearrangements. External conditions conducive to spreading are further provided by physical factors (for example, fluid pressure and stiffness) and surrounding cells (for example, cancer-associated fibroblasts and endothelial cells) in the tumour microenvironment. Mechanical stimuli from the tumour microenvironment promote pro-metastatic conditions by activating Yes-associated protein 1 (YAP)–transcriptional co-activator with PDZ-binding motif (TAZ) in cancer associated fibroblasts, favouring cancer cell invasion. Further, paracrine factors secreted by endothelial cells may reduce PHGDH levels in cancer cells, potentiating cell migration and invasion. Intravasation (part b) and circulation (part c). Circulating tumour cells (CTCs) and their clusters have a short half-life in circulation, due to hostile conditions, including physical forces (that is, shear stress) and anoikis. CTCs can escape the immune system via downregulation of major histocompatibility complex class I (MHC-I), expression of immune checkpoint molecules (for example, programmed cell death protein 1 (PD1) ligand 1 (PDL1) and CD47) or through support from platelets and neutrophils. Cell-intrinsic factors (for example, expression of anti-apoptotic factors) enhance CTC survival and successful transit, while circadian rhythmicity (and related hormone fluctuations) dictates the timing of CTC intravasation events, reaching a peak during the rest phase. d, Extravasation. The efficiency of extravasation relies upon the expression of adhesion molecules (for example, CD44, mucin 1 (MUC1) and sialyl-Lewis A (sLeA)/sialyl-Lewis X (sLeX), chemokine release, physical properties (for example, CTC cluster size and deformability) and supporting cells (for example, neutrophils via the formation of neutrophil extracellular traps (NETs)). Signalling via transforming growth factor-β (TGFβ)/SMAD family member 3 (SMAD3) leads to the upregulation of various adhesion-related molecules and facilitates CTC vascular adhesion. e, Successful homing into a new environment is dependent on niche factors (that is, various organ-specific cell types), but is also influenced by the preset metastatic potential of CTCs. f, CTCs may spread from the primary tumour or from metastatic lesions to either seed new metastasis (metastasis-to-metastasis dissemination) or return to the primary tumour site (tumour self-seeding). DTC, disseminated tumour cell; HIFPH2, hypoxia-inducible factor prolyl hydroxylase 2; NK natural killer; N-WASP, neural Wiskott–Aldrich syndrome protein.