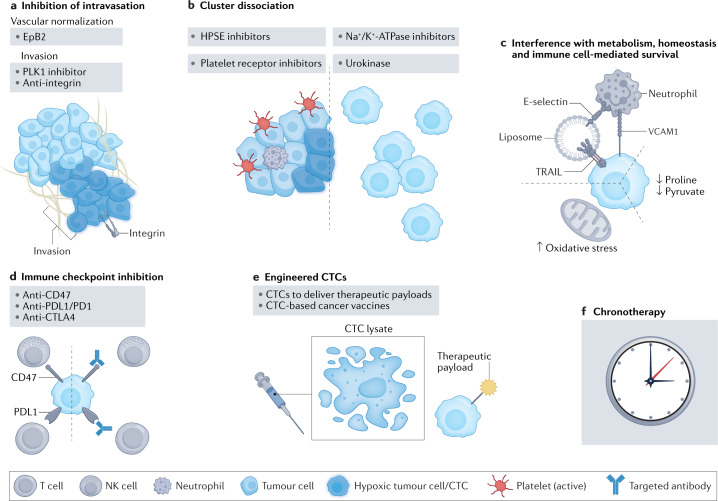
Fig. 4. Circulating tumour cell-targeting strategies.
Outlined here are various potential circulating tumour cell (CTC)-targeting strategies that are proposed on the basis of recent experimental work. a, Inhibition of cancer cell intravasation via normalization of the hypoxic tumour microenvironment; for example, by ephrin B2 Fc chimaera protein (EpB2) treatment leading to modulation of vascular endothelial growth factor receptor (VEGFR) signalling and vascular normalization, blockade of intravasation-relevant proteins (for example, Polo-like kinase (PLK1) inhibition) or blocking of cellular interactions between cancer and endothelial cells (for example, integrin-targeted antibodies). b, Dissociation of CTC clusters or prevention of their formation, for example via Na+/K+-ATPase inhibition, heparanase (HPSE) inhibition, stimulation with the urokinase-type plasminogen activator (urokinase) or inhibition of platelet receptors on CTCs. c, Targeting CTC survival via metabolic interference by increasing oxidative stress and inhibition of pyruvate or proline metabolism, or by use of E-selectin/tumour necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL)-coated nanoliposomes that mimic the activity of natural killer (NK) cells. d, ‘Demasking’ CTCs for immune clearance by targeting immune evasion via immune checkpoint inhibitors against programmed cell death protein 1 (PD1) and PD1 ligand 1 (PDL1), cytotoxic T lymphocyte-associated antigen 4 (CTLA4) or CD47. e, Use of engineered CTCs as therapeutic vehicles (for example, prodrug conjugates) or CTCs for tumour vaccine development. f, CTC-based chronotherapy (that is, delivering treatments to be maximally effective at the times of greatest CTC production). VCAM1, vascular cell adhesion molecule 1.