Figure 4: CryoEM identifies β-tubulin folding intermediates in ATP-closed TRiC chamber.
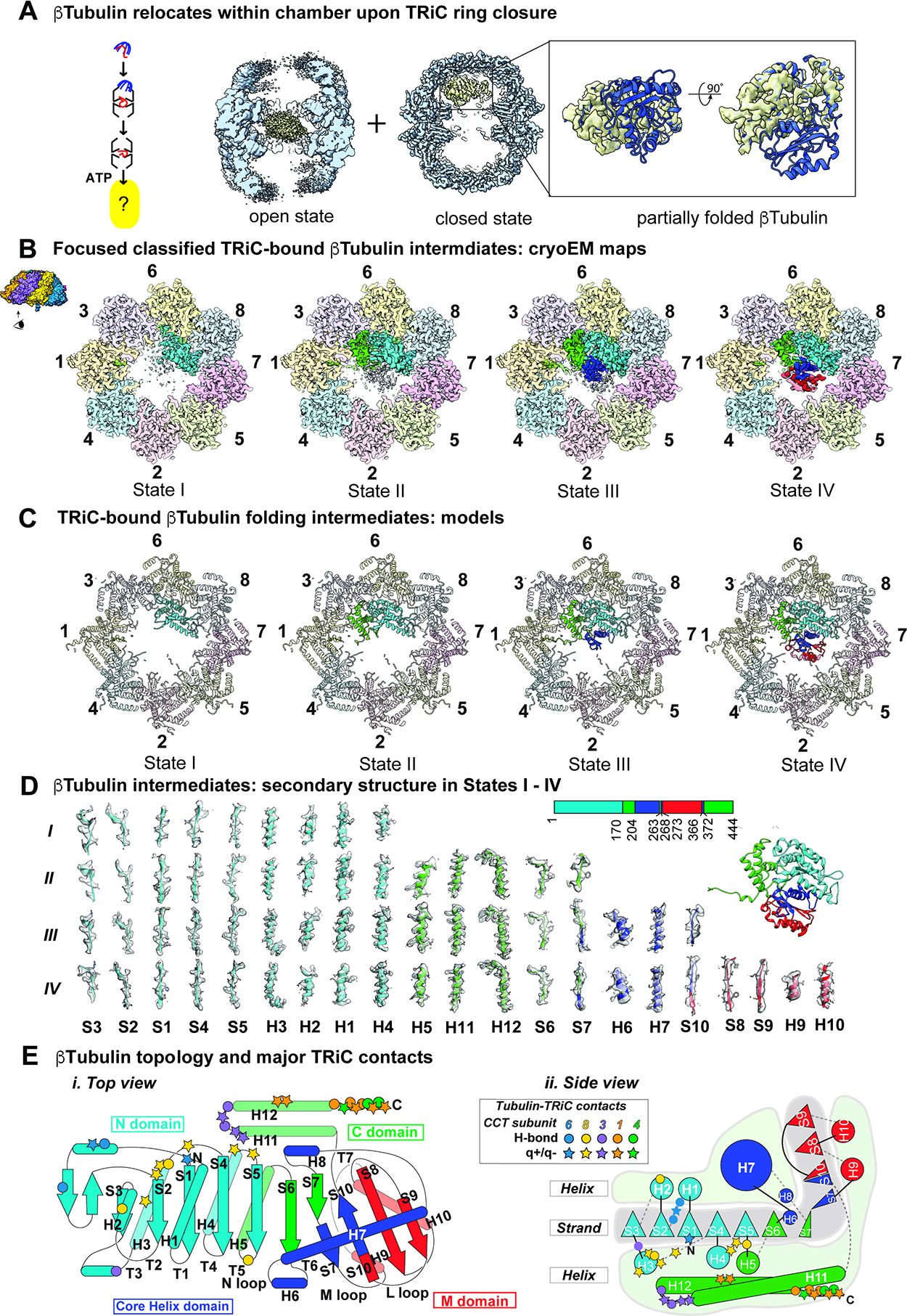
(A) CryoEM consensus maps of open and closed TRiC:β-tubulin indicates dramatic repositioning of β-tubulin density. (B) Focused classification on β-tubulin in the closed state identified four distinct states I-IV that could be separated by the amount of progressively folded β-tubulin relative to the final state of β-tubulin. (C) Atomic models of TRiC bound β-tubulin folding intermediates. (D) Local resolvability of secondary structure elements in β-tubulin folding intermediates reveals progressive formation of folded domains. (E) Cartoon illustration of β-tubulin topology highlighting major contacts with TRiC subunits.
