Figure 1.
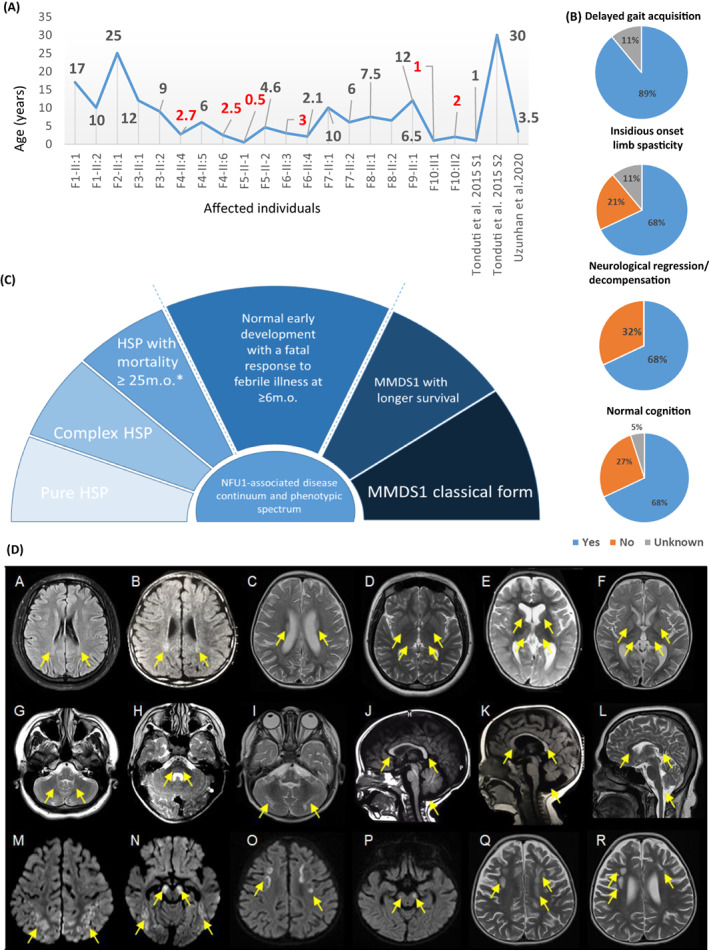
Clinical features of the individuals reported in this study and NFU1‐associated phenotypic continuum. (A) Ages of the affected individuals at the study recruitment. (B) Clinical features of the present cohort. (C) NFU1‐associated phenotypic continuum. HSP, hereditary spastic paraplegia. (D) Representative brain MRI features of the present cohort. Individual F1‐II:2 (A, D, G, L), individual F4‐II:5 (B), individual F10‐II:1 (C, F, I, M, N), individual F5‐II:2 (E, K), individual F7‐II:1 (H), individual F4‐II:6 (J), and individual F10‐II:2 (O, P, Q, R). T2/FLAIR hyperintense signal involving the bilateral posterior centrum semiovale (Q), corona radiata, and periatrial regions (A‐C). T2 hyperintense signal involving the bilateral thalami and basal ganglia (D‐F), pons, and cerebellum (G‐I). Hypoplastic corpus callosum and mega cisterna magna (J‐L). Bilateral cerebral white matter volume loss (A‐F, Q, R). Areas of restricted diffusion involving the bilateral subcortical white matter and cerebral peduncles (M‐P). Areas of cystic degeneration/leukomalacia in the white matter of the bilateral frontal lobes (Q, R). Vermian hypoplasia (K).
