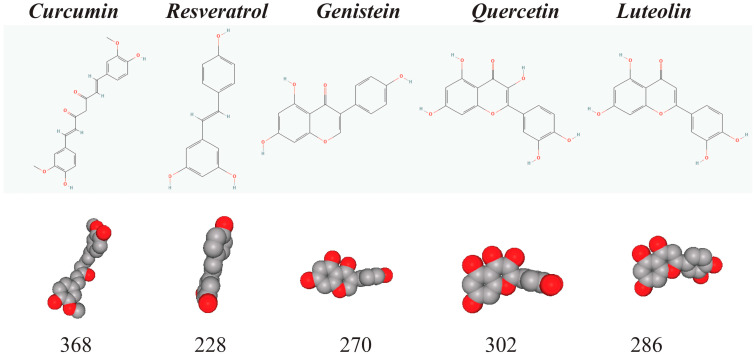
Figure 1.
Chemical structures of curcumin, resveratrol, genistein, quercetin, and luteolin. This figure shows chemical structures (top row), space-filling models (medium row), and exact mass (bottom row) of curcumin, resveratrol, genistein, quercetin, and luteolin. Curcumin is a diarylheptanoid from the group of curcuminoids, while resveratrol is a 3,4′,5-trihydroxy-trans-stilbene. Genistein, quercetin, and luteolin are flavonoids derived from a 15-carbon skeleton with two aromatic phenyl rings (A and B). Flavonoids also contain a heterocyclic pyran ring (C) with an embedded oxygen. These are small molecules—with molecular weight between 228 and 368—that are aromatic. For example, curcumin and resveratrol contain two aromatic phenyl rings. Note that such natural phenols are chemically active in many types of organic reactions that are common in a living cell, such as free radical chemistry, nucleophilic addition, or metal ion interactions [8].