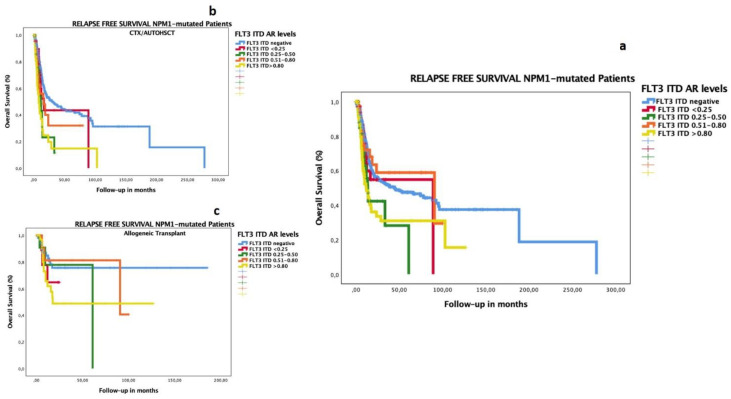
Figure 2.
Impact on RFS according to FLT3–ITD AR in patients with AML co-occurring with mutated NPM1. The RFS median for NPM1-mutated with FLT3–ITD-negative group was 44.75 months (CI 17.4.0–72.10); that with FLT3–ITD AR <0.25 was 89.18 months (not reached); that with FLT3–ITD AR 0.25–0.50 was 13.83 months (10.9–16.7); that with FLT3–ITD AR 0.51–0.80 was 90.75 months (0–185.7); that with FLT3–ITD AR > 0.8 was 12.13 months (7.3–16.9) (p < 0.001) (a). We analyzed the impact of the test of equality of survival distribution for different levels of FLT3–ITD AR, adjusted for consolidation treatment, namely CTX/auto-HSCT (b) or allogeneic transplantation (c): the RFS median in the FLT3–ITD-negative group was 29.8 months vs. not reached (NR); 16.8 months vs. NR in FLT3–ITD AR < 0.25; 11.8 vs. 61.0 months in FLT3–ITD AR 0.25–0.50; 15.9 vs. 90.7 months in FLT3–ITD AR 0.51–0.80; and 8.4 vs. 17.8 months in FLT3–ITD AR > 0.80 (p < 001), respectively. The group consolidated with auto-HSCT or chemotherapy included 161 patients: FLT3 ITD-negative (138 cases, 60% censored), FLT3 AR < 0.25 (5 cases, 20% censored), FLT3 AR 0.25–0.50 (7 cases, 29% censored), FLT3 AR 0.501–0.80 (3 cases, 67% censored), and FLT3 AR > 0.8 (8cases, 25% censored). The group consolidated with allo-HSCT included 143 patients: FLT3 ITD-negative (58 cases, 78% censored), FLT3 AR < 0.25 (11 cases, 73% censored), FLT3 AR 0.25–0.50 (11 cases, 45% censored), FLT3 AR 0.501–0.80 (26 cases, 81% censored), and FLT3 AR > 0.8 (37cases, 62% censored).