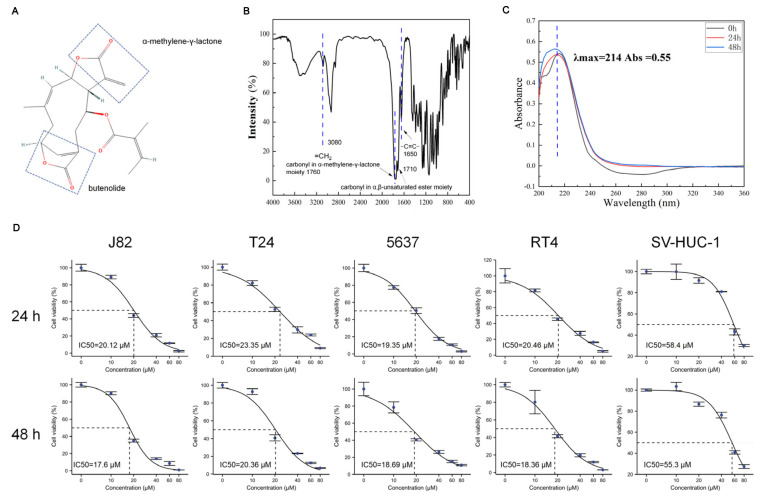
Figure 1.
Chemical structure, stability, and efficacy of scabertopin against bladder cancer cells. (A). Chemical structure scabertopin. The two boxes show the structure of the molecule with the drug activity of α-methylene-γ-lactone and butenolide. (B). Infrared spectrum of scabertopin. The stretching vibration of =CH2 exists at 3080 cm−1, and the carbonyl stretching vibration of α-methylene-γ-lactone structure exists at 1760 cm−1. The peak at 1710 cm−1 is the carbonyl stretching vibration of another non-lactone α,β-unsaturated ester, and the C=C stretching vibration exists at 1650 cm−1. (C). The UV absorption peak of scabertopin in the DMEM medium at 0, 24, and 48 h. (D). 24 and 48 h IC50 of bladder cancer cell lines (J82, T24, 5637, and RT4) and human ureteral epithelial immortalized cells (SV-HUC-1) (n = 4).