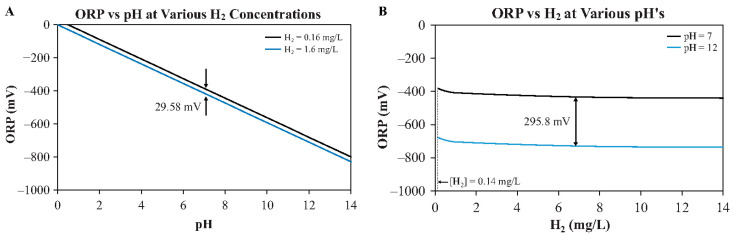
Figure 1.
Changes in ORP as a function of either pH (A) or H2 concentration (B). Temperature held constant at 25 °C. Note that although the concentration of H2 at the standard ambient temperature and pressure is 1.6 mg/L, it may be higher than this at lower temperatures and/or higher pressures accordingly to Henry’s law (C = P/KH; C is concentration, P is pressure, and KH is Henry’s solubility constant for the specific gas at a given temperature).