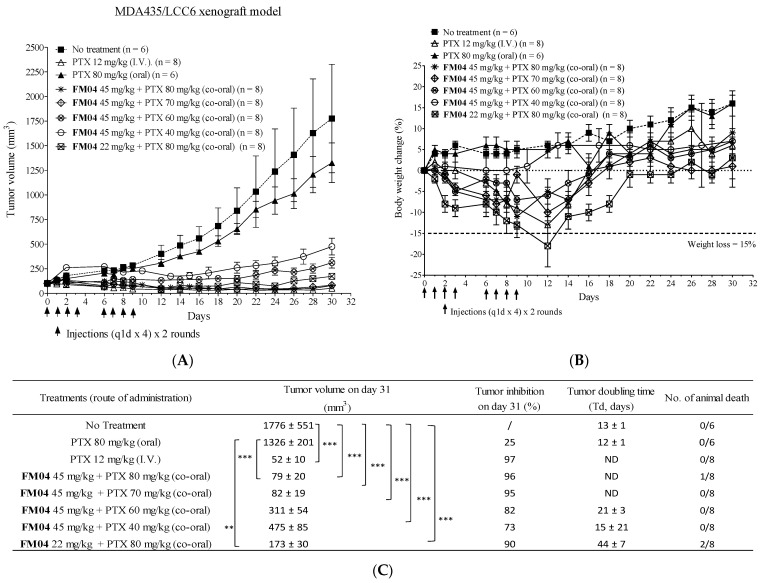
Figure 8.
In vivo anti-tumor efficacy of oral co-administration of FM04 with PTX in human melanoma MDA435/LCC6 xenograft. Balb/c nude mice were subcutaneously xenografted with LCC6. The treatment groups included (1) control: no treatment, (2) PTX 12 mg/kg (I.V.) alone, (3) PTX 80 mg/kg (oral) alone, (4) co-treatment: FM04 45 mg/kg + PTX 80 mg/kg (co-oral), (5) co-treatment: FM04 45 mg/kg + PTX 70 mg/kg (co-oral), (6) co-treatment: FM04 45 mg/kg + PTX 60 mg/kg (co-oral), (7) co-treatment: FM04 45 mg/kg + PTX 40 mg/kg (co-oral) and (8) co-treatment: FM04 22 mg/kg + PTX 80 mg/kg (co-oral). (A) Tumor volume and (B) body weight were monitored throughout the treatment. The treatment was given to mice every day for 4 times and 2 rounds [(q1d × 4) x 2 rounds] and indicated as an arrow. The values were presented as mean ± standard error of mean (n = 6–8 mice per group). (C) Tumor inhibition % on day 30 and tumor doubling time after each treatment were determined. Number of animal deaths was monitored during the treatment. ND = tumor-doubling time of co-treatment groups FM04 at 45 mg/kg + PTX at 80 or 70 mg/kg or PTX alone at 12 mg/kg (I.V.) cannot be determined as tumor volume was smaller than the initial tumor volume 100 mm3. For the tumor volume at each time point, statistical analysis was conducted by one-way-ANOVA between co-treatment group and control or PTX alone treatment. ** p < 0.01; *** p < 0.001. /: not applicable.