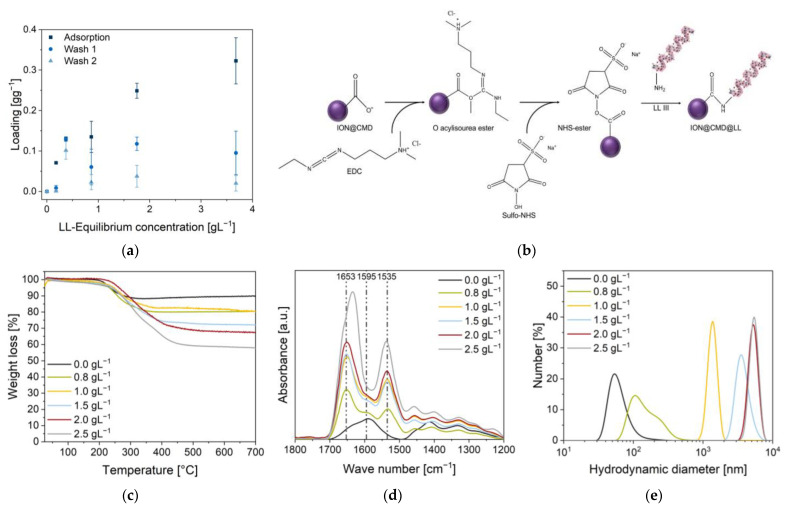
Figure 6.
(a) Adsorptions of LL at pH 7.4 in 50 mM of PBS buffer and 1 g L−1 of ION@CMD12.5. Covalent binding of cationic peptide LL (0.0 g L−1–2.5 g L−1 input) to the surface of the ION@CMD12.5 particles. (b) EDC reacted with the free carboxyl group of CMD to form an unstable O-acylisourea ester intermediate. Sulfo-NHS was added to the reaction to form a more stable NHS ester, which reacts slowly with primary amines of LL to form stable amid bonds. LL was created with BioRender.com. (c) TGA measurements until 700 °C. (d) FT-IR spectra of the ION@CMD and ION@CMD@LL particles (24 scans) with labeled characteristic LL bands at 1653 cm−1 and 1535 cm−1 and the characteristic CMD band at 1595 cm−1. (e) Hydrodynamic diameters of the unloaded (ION@CMD) and loaded particles in an aqueous medium (pH = 7–8).