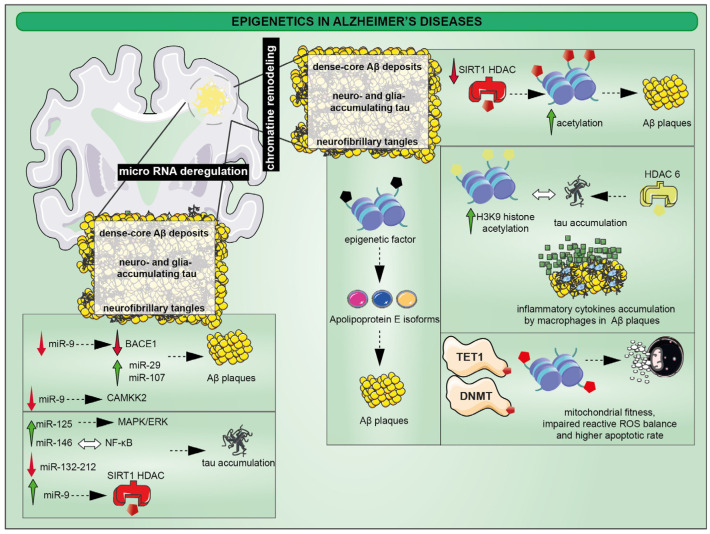
Figure 2.
Accumulation of abnormal neurotic plaques and neurofibrillary tangles are the main pathological traits of AD. The first is related to the incorrect sequential cleavage of the amyloid precursor generating the fibrous protein enriched of β-sheet secondary structures. The second is the result of hyperphosphorylation of microtubule-associated tau protein in neurons leading to cytoskeletal changes. Among epigenetic changes, the acetylation processes lead to several effects. The reduction of Sirt1, a class III HDAC, determines a lack of histone deacetylation with downstream effects resulting in the development of Aβ plaques. However, inflammatory processes triggered by reactive microglia in plaques orchestrates tau hyperphosphorylation, where H3K9 acetylation by HDAC6 activity has been found to be implicated in the epigenic changes. Moreover, aberrant DNA methylation, especially DNMTs and TET, are associated to mitochondrial dysfunction causing the homeostatic loss of ROS balance which in turn is responsible for the apoptotic death of neurons. Finally, epigenetic changes also drive the formation of apolipoprotein E isoforms that show increased production and reduced degradation capacity of Aβ plaques; miR-9 is one the main miRNAs involved in AD pathogenesis, since its reduction determines a decrease of BACE1 and the activation of CAMKK2 which are responsible for Aβ plaques and p-tau, respectively. On the other hand, its upregulation control SIRT1 activity proves the correlation between miRNA and chromatin remodeling in AD pathological traits; MAPK/ERK activation is related to miR-125b for p-tau formation, whereas miR-146 expression is strictly linked to NF-κB. Other miRNAs, such as miR-132/-212 and the interaction between miR-29 and miR-107, directly correlate with Aβ plaques and p-tau accumulation.