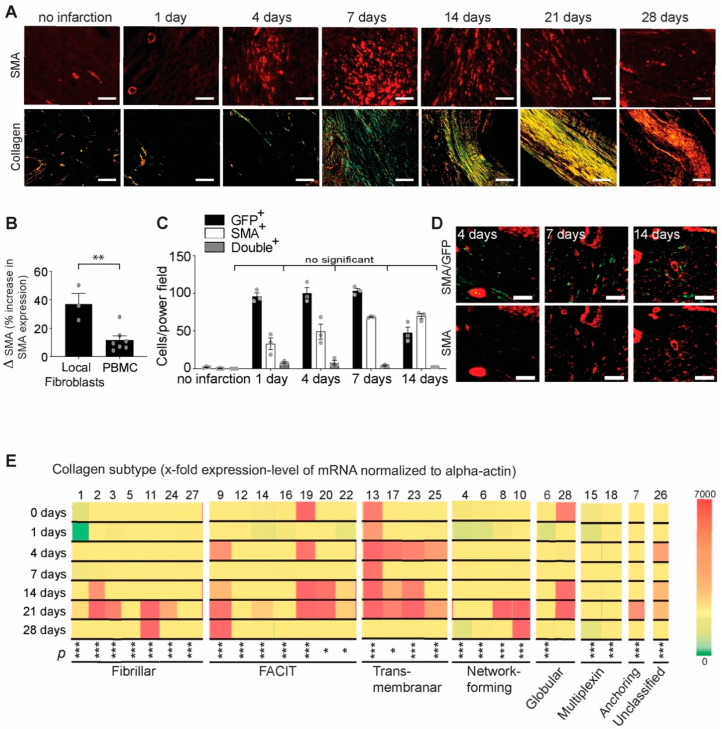
Figure 1.
Characterization the origin and collagen subtype expression of myofibroblasts after myocardial infarction. (A) Time course of alpha smooth muscle actin staining (red, upper panel; scale bar, 50 µm) and Sirius red staining (lower panel, scale bar 200 µm) after myocardial infarction. (B) Percentage of alpha smooth muscle actin-positive (myofibroblasts) cells differentiated from cardiac fibroblasts and blood mononuclear cells (PBMC). As determined by α-SMA staining, local cardiac fibroblasts have an increased potential to differentiate towards myofibroblasts under TGF-ß1 stimulation and under hypoxic conditions compared with blood cells (** p < 0.01, t-test, n = 3–7 mice). (C) GFP-positive bone marrow reconstitution of lethally irradiated mice reveals that local differentiated myofibroblasts constitute most of the cells responsible for extracellular matrix synthesis; GFP/SMA double-positive cells (gray columns) are not significant compared with the control (two-way ANOVA followed by Tukey’s post hoc test, n = 3 mice per group). (D) Selected representative images of GFP/SMA double-positive cells (upper panel, yellow) and α-SMA cells (lower panel, red) at the time points characteristic of increased presence of myofibroblasts (scale bar, 50 µm). (E) mRNA expression levels of different collagen subtypes at different time points after myocardial infarction (normalized to α-actin) (* p < 0.05, *** p < 0.001, one-way ANOVA for each collagen subtype followed by Tukey’s post hoc test, n = 3 mice per group).