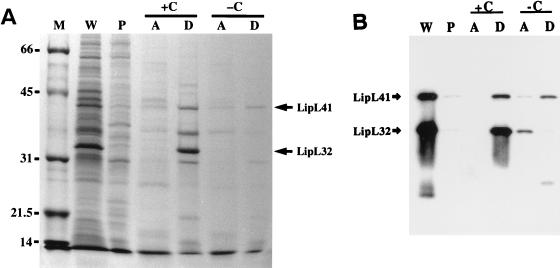
FIG. 4.
Behavior of LipL32 and other leptospiral proteins in Triton X-114. Triton X-114 fractions of L. kirschneri organisms were separated by SDS-PAGE and stained with Coomassie brilliant blue (A) or probed with either a combination of LipL32 and LipL41 antisera (B). Fractions analyzed were the whole organism (W), Triton X-114-insoluble pellet (P), and aqueous-phase (A) or detergent-phase (D) material with (+C) or without (−C) 20 mM CaCl2. LipL32, but not LipL41, requires CaCl2 to avoid proteolytic degradation during Triton X-114 phase partitioning.