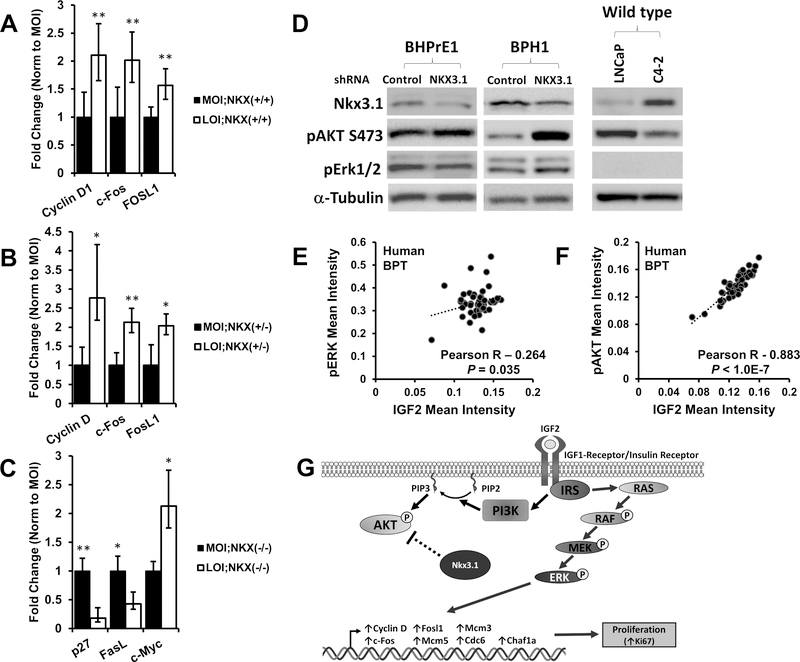
Figure 5. Transcription of p-ERK and p-AKT effector genes are altered in Igf2 LOI prostates, IGF2 correlates with p-ERK and p-AKT in human prostate tissues.
Gene transcription was analyzed in MOI and LOI mouse prostate tissues (Minimum n=5 each group). A, Increased mRNA expression of p-ERK target genes Cyclin D1 (P=0.003), c-Fos (P=0.003), and Fosl1 (P=0.009) was demonstrated in LOI;Nkx3.1+/+ prostates. B, CyclinD1 (P=0.029), c-Fos (P=0.004), and Fosl1 (P=0.017) were significantly increased in LOI;Nkx3.1+/− prostates. C, Altered mRNA expression of p-AKT target genes p27 (P=0.0081), Fasl (P=0.033), and c-Myc (P=0.044) in LOI;Nkx3.1−/− prostate tissues D, Western blot of NKX3.1 knockdown. An siRNA for NKX3.1 (and non-silencing control) was transfected into the BHPrE1, a nontumorigenic human epithelial cell line, and BPH1 cells and protein lysates analyzed. Knockdown of NXX3.1 results in a pAKT increase in BHPrE1 and BPH1 cells and pERK downregulation in BHPrE1. An inverse correlation between NKX3.1 expression and pAKT is noted in LNCaP and its derivative C4–2. E, Quantitative IHC in human prostate tissues demonstrates a significant correlation between IGF2 and p-ERK expression in benign prostate tissues (BPT). F, IGF2 and p-AKT protein expression strongly correlate in human benign prostate tissues. Correlation coefficient and P-value calculated using Pearson’s product moment correlation (BPT, n=48). G, In LOI affected prostates containing at least one Nkx3.1 allele, proliferation is predominantly mediated via MAPK/ERK signaling (right). In contrast, Nkx3.1 negatively regulates AKT signaling in Nkx3.1+/n animals, and Nkx3.1−/− prostates exhibit increased p-AKT.