Figure 1. Enrollment, Randomization, and Follow-up.
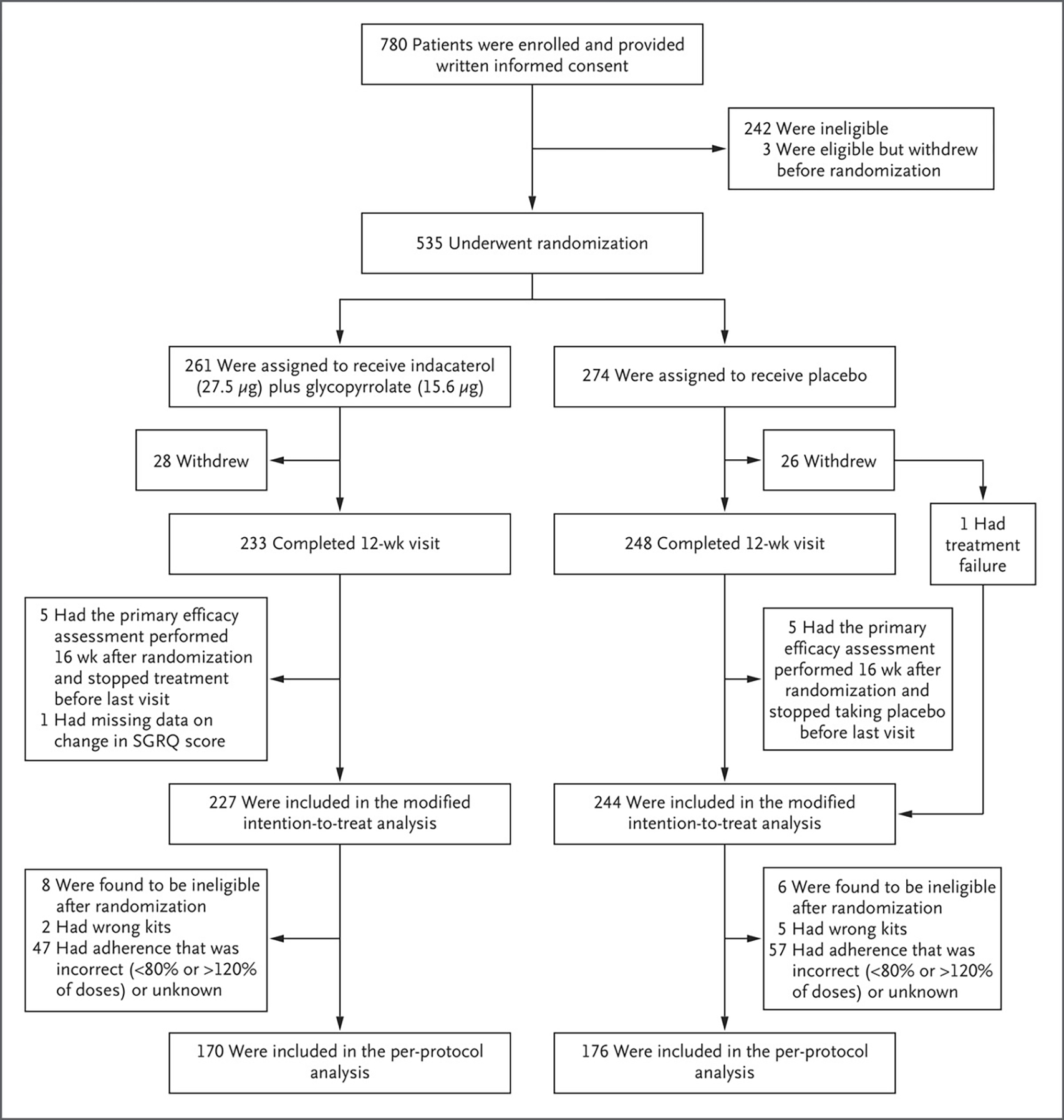
Participants were randomly assigned to receive indacaterol (27.5 μg) plus glycopyrrolate (15.6 μg) or placebo twice daily for 12 weeks. The modified intention-to-treat population excluded participants who had neither treatment failure nor week 12 data for the primary outcome. The per-protocol population excluded participants who had a protocol deviation or incorrect or unknown adherence. SGRQ denotes St. George’s Respiratory Questionnaire.
